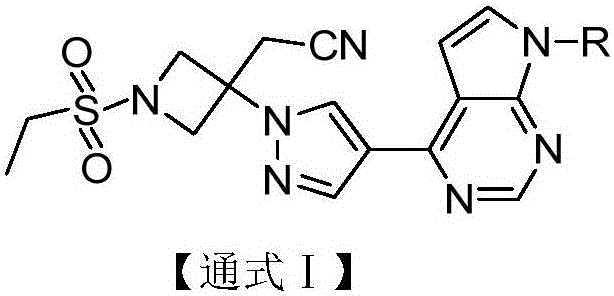
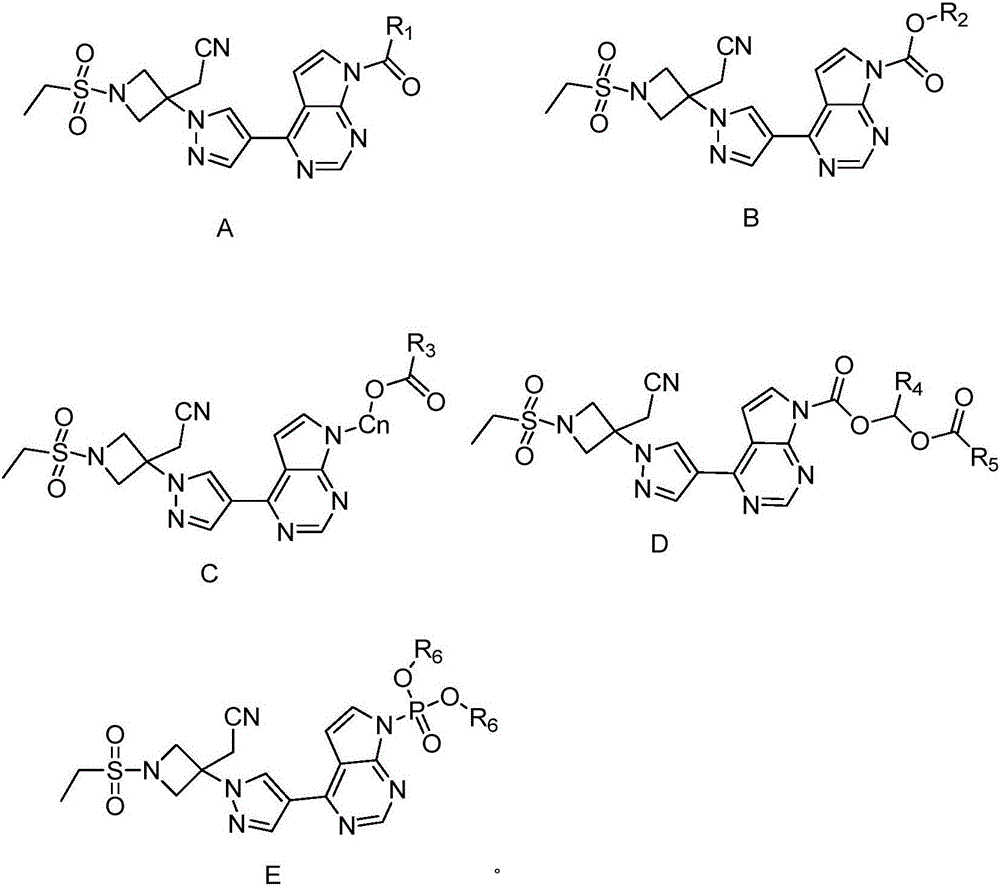
Pyrrolomiazine compound and preparation method and application thereof
A pyrrolopyrimidine and compound technology, which is applied in the fields of organic compound synthesis and pharmaceutical application, can solve the problem of no modification of pyrrole ring amino group, achieve good solubility, high bioavailability, and enhance drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of Compound A-1
[0046]
[0047] Add compound 1 (150 mg, 0.40 mmol, 1 equiv) into a mixed solvent of dichloromethane and acetonitrile to dissolve it, then add triethylamine (140 mL, 2.5 equiv), react the reaction system in an ice-water bath for 15 minutes, and slowly add ethyl Acyl chloride (0.044mL, 0.60mmol, 1.5equiv), slowly warmed up to room temperature, stirred overnight. The filtrate was concentrated, mixed with silica gel, and subjected to direct column chromatography (dichloromethane:methanol=50:1) to obtain compound A-1 (106 mg), with a yield of 64%. l H-NMR (300MHz, DMSO-d6) δ: 9.01 (s, 1H), 8.96 (d, J = 5.3, 1H), 8.52 (s, 1H), 8.13 (d, J = 4.1, 1H), 7.43 ( m,J=5.1,1H),4.61(d,J=9.2,2H),4.25(d,J=9.2,2H),3.69(s,1H),3.25(m,J=8.6,2H),3.00 (s,3H),1.24(m,J=4.9,3H)
Embodiment 2
[0048] Example 2 Preparation of Compound A-2
[0049]
[0050] Compound 1 (150 mg, 0.40 mmol, 1 equiv) was dissolved by adding a mixed solvent of dichloromethane and acetonitrile, then triethylamine (140 mL, 2.5 equiv) was added, and the reaction system was reacted in an ice-water bath for 15 minutes, and slowly added Isobutyryl chloride (0.084 mL, 0.60 mmol, 1.5 equiv), warmed up slowly to room temperature, and stirred overnight. The filtrate was concentrated, mixed with silica gel, and subjected to direct column chromatography (dichloromethane:methanol=50:1) to obtain compound A-2 (128 mg), with a yield of 72%. l H-NMR (300MHz, DMSO-d6) δ: 8.93(s, 1H), 8.44(s, 1H), 8.31(s, 1H), 8.01(d, J=4.1, 1H), 6.87(d, J= 4.1,1H),4.62(m,J=6.1,2H),4.25(d,J=9.4,2H),3.41(s,2H),3.09(m,J=7.4,2H),1.41(d,J =7.4,3H),1.38(t,J=4.9,6H)
Embodiment 3
[0051] Example 3 Preparation of Compound A-3
[0052]
[0053] Compound A-3 (125 mg) was synthesized in the same manner as compound A-1 in Preparation Example 1 except that n-butyryl chloride was used instead of acetyl chloride, with a yield of 70%. l H-NMR (300MHz, CDCL3) δ: 8.93(s, 1H), 8.44(s, 1H), 8.31(s, 1H), 8.06(d, J=4.1, 1H), 6.86(d, J=4.2, 1H), 4.64(d, J=9.5, 2H), 4.25(d, J=9.5, 2H), 3.53(t, J=7.3, 2H), 3.41(s, 2H), 3.09(m, J=7.4 ,2H),1.88(m,J=7.4,2H),1.42(t,J=7.4,3H),1.11(t,J=7.4,3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com