The preparation method of the key intermediate 1 for the synthesis of baricitinib
A technology of baricitinib and intermediates, which is applied in the field of preparation of key intermediate 1, can solve the problems of high cost and cumbersome operation, and achieve the effects of low cost, simple purification and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
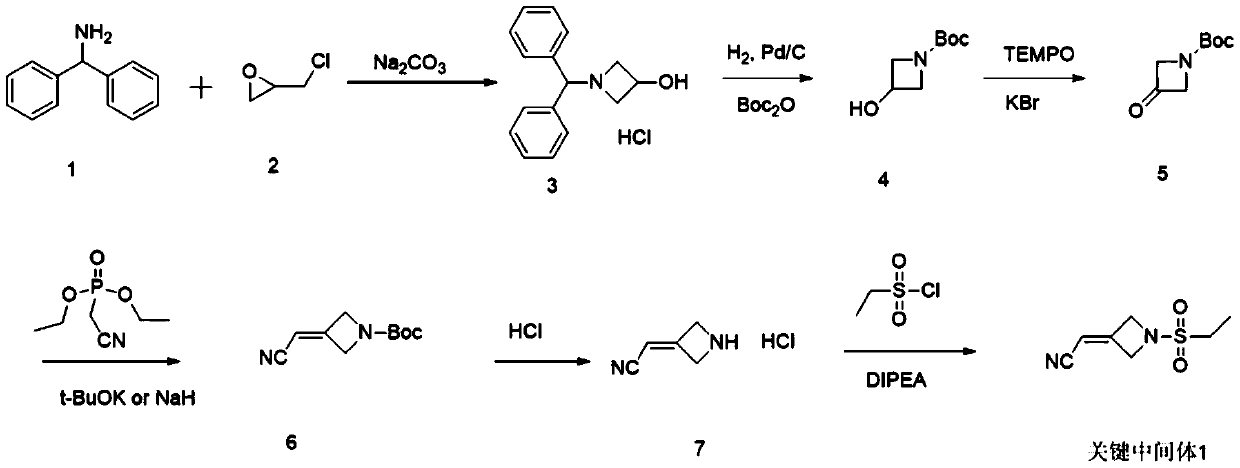
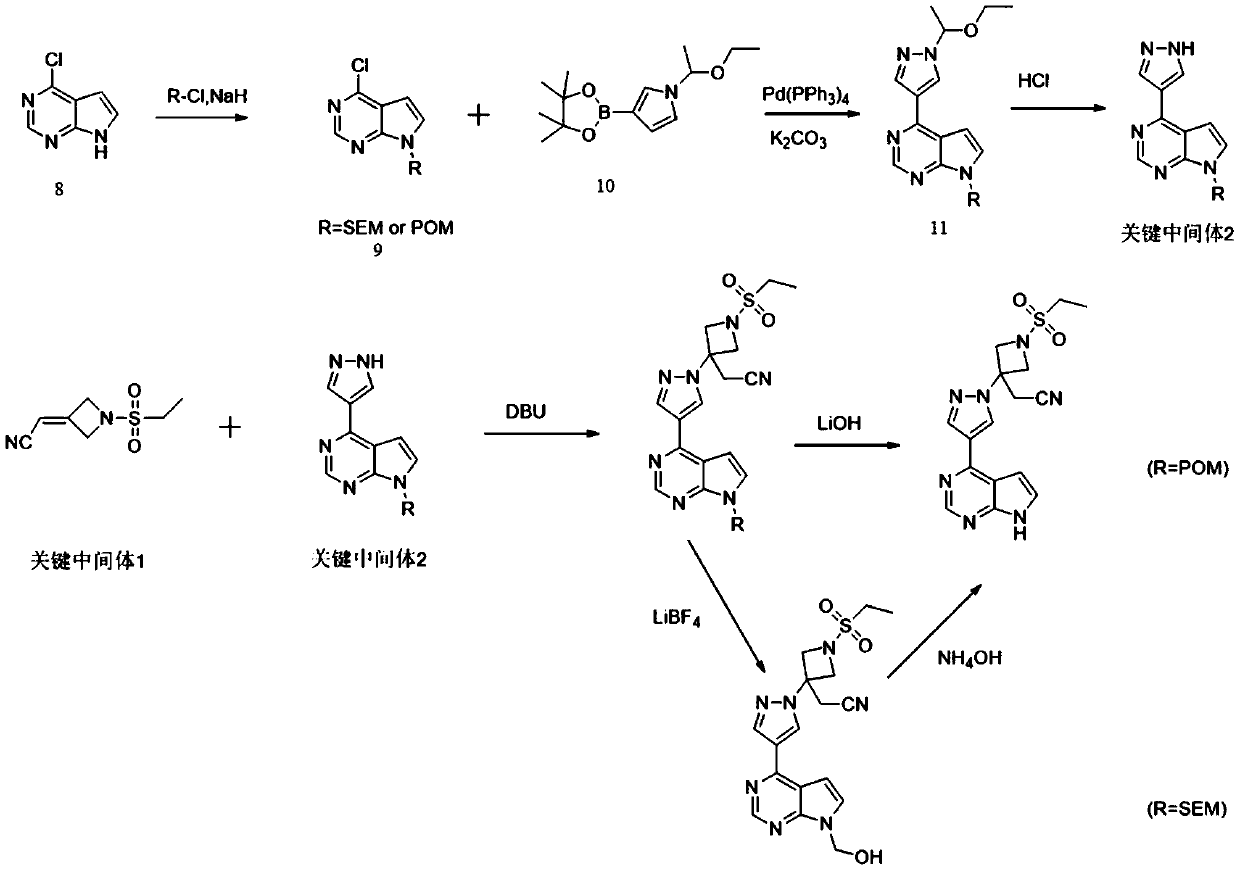
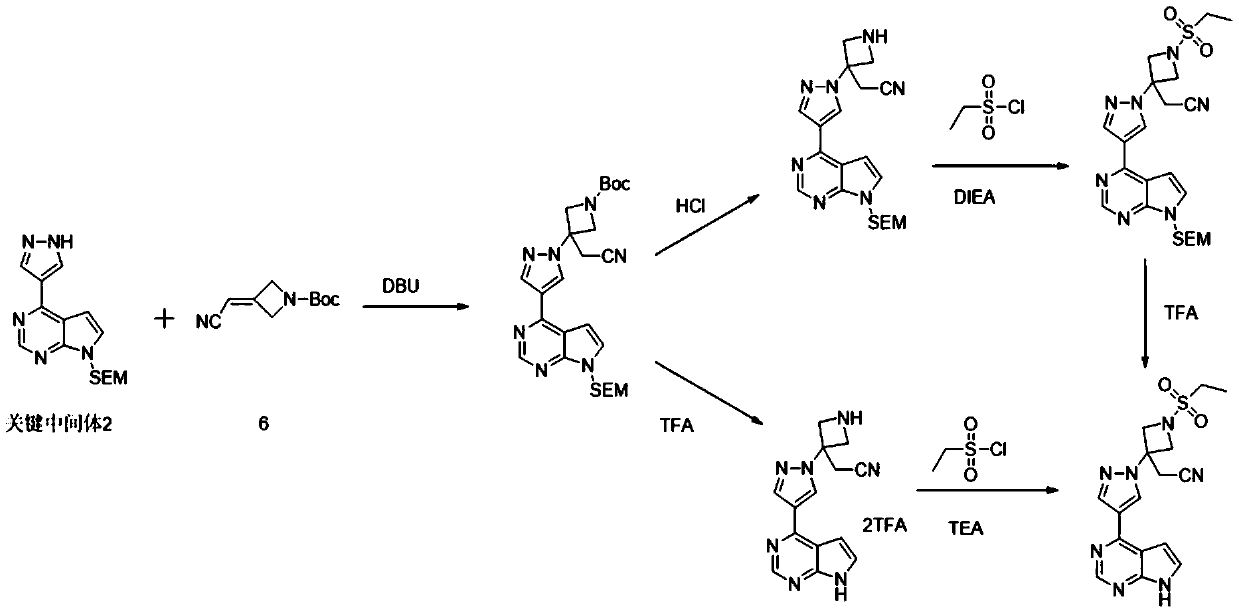
[0030] A preparation method for the key intermediate 1 of baricitinib synthesis, which comprises the following steps:
[0031] (1) Synthesis of intermediate B: 1,3-dibromo-2,2-dimethoxypropane (SM), ethanesulfonamide (SM2) reacted with ring-closing reaction under alkaline conditions, and then under acidic conditions Next, acetal deprotection obtains intermediate B, and the structural formula of said intermediate B is:
[0032]
[0033] (2) Synthesis of the key intermediate 1: the intermediate B obtained in step (1) is reacted with diethyl cyanomethylphosphonate under alkaline conditions to obtain the key intermediate 1, the key intermediate 1 The structural formula is:
[0034]
[0035] Wherein, the specific operation method of step (1) is: under the protection of nitrogen, add ethanesulfonamide, alkaline reagent A and organic solvent A into the reaction flask, stir for 30-40min; then add 1,3-dibromo-2 , 2-dimethoxypropane (SM), stirred for 30-40min; then the reaction ...
Embodiment 1
[0046] 1.1 Synthesis of Intermediate B
[0047]Under the protection of nitrogen, in a 5L three-necked flask, add the starting materials ethanesulfonamide (163.5g, 1.5mol), anhydrous potassium carbonate (275.8g, 2.0mol) and anhydrous DMF (1L), stir for 30min, then add 1,3-dibromo-2,2-dimethoxypropane (SM) (259.9g, 1.0mol), stirred for 30min, then raised the temperature of the reaction system to 95-100°C, and reacted at this temperature for 20-22h , and then cooled to room temperature. Afterwards, use 0.1N HCl to adjust the pH to 2~3, and stir thoroughly under this condition for 2-3h, then add 2L of water to the system, and a large amount of off-white solid precipitates, which is filtered and dried to obtain 134.1g of solid, with a yield of 82.3 %.
[0048] NMR analysis:
[0049] 1 H-NMR (400MHz, DMSO-d6): 5.12(m,2H), 5.08(m,2H), 3.12(q,2H), 1.44(t,3H).
[0050] 1.2 Synthesis of key intermediate 1
[0051] Under the protection of nitrogen, add diethyl cyanomethylphosphonat...
Embodiment 2
[0055] 2.1 Synthesis of Intermediate B
[0056] Under nitrogen protection, in a 5L three-necked flask, add the starting materials ethanesulfonamide (163.5g, 1.5mol), anhydrous potassium carbonate (275.8g, 2.0mol) and anhydrous THF (1L), stir for 30min, and then add SM (259.9g, 1.0mol), stirred for 30min; then the reaction system was heated up to 60-65°C, and reacted for 35-40h under this temperature condition; then cooled to room temperature, adjusted to PH=4~5 with 0.1N HCl, in Stir well under this condition for 2-3h; then add 2L of water to the system, a large amount of off-white solid precipitates, filter and dry to obtain 96.5g of solid with a yield of 59.2%.
[0057] 2.2 Synthesis of key intermediate 1
[0058] Under nitrogen protection, in a 3L three-necked flask, add diethyl cyanomethylphosphonate (265.6g, 1.5mol) and anhydrous DMF (300ml), stir to dissolve; then lower the system temperature to -10~0℃ , under this condition, slowly add THF (200ml) solution containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com