Baricitinib cream and preparation method and application thereof
A technology of baricitinib and Ni cream, which is applied in the direction of ointment delivery, pharmaceutical formula, emulsion delivery, etc. It can solve the problems such as difficult to obtain cream with particle size distribution, and achieve the goal of being suitable for industrial production and having good coating performance , the effect of not easy to separate oil and water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Recipe for Cream 1
[0061] The composition of the preparation prescription is the content of components in every 100 grams of cream:
[0062] Baricitinib 250 mg;
[0063] Polyethylene glycol-7 stearate (tefose 63) 22 grams;
[0064] Purified water was added to make up to 100 g.
[0065] making process:
[0066] (a) Polyethylene glycol-7 stearate is melted at 60°C;
[0067] (b) heating the purified water to 60°C;
[0068] (c) under continuous stirring at 800 rpm, transfer the hot purified water into the polyethylene glycol-7 stearate liquid, and stir for 30 minutes;
[0069] (d) Turn the mixture to 30°C, stir continuously at 800 rpm for 15 minutes, add baricitinib and stir evenly;
[0070] (e) filling to obtain baricitinib emulsifiable cream.
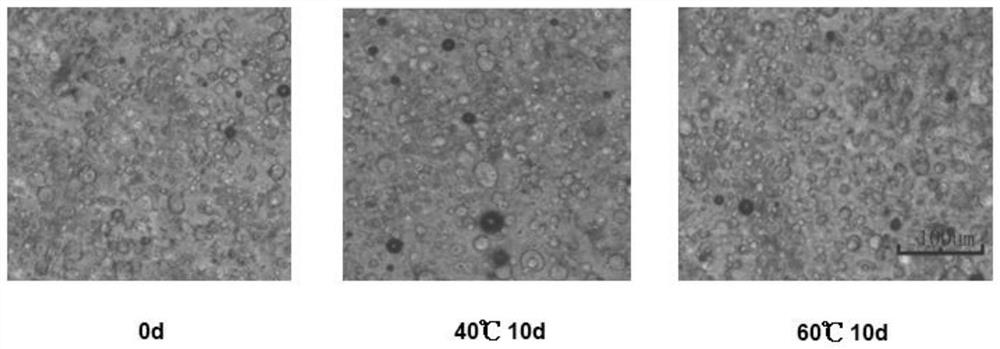
[0071] The morphology of the obtained cream was observed under an optical microscope, and the cream was placed at 40 degrees Celsius and 60 degrees Celsius for 10 days, and cooled to room temperature after taking it out to...
Embodiment 2
[0073] Recipe for Cream 2
[0074] The composition of the preparation prescription is the content of components in every 100 grams of cream:
[0075] Baricitinib 250 mg;
[0076] Polyethylene glycol-7 stearate (tefose 63) 18 grams;
[0077] 5.5 grams of liquid paraffin;
[0078] Phenoxyethanol 0.45 g;
[0079] Appropriate amount of sodium hydroxide;
[0080] Purified water was added to make up to 100 g.
[0081] making process:
[0082] (a) Polyethylene glycol-7 stearate and liquid paraffin are melted at 60°C;
[0083] (b) heating purified water to 60°C, adding ethyl p-hydroxybenzoate to dissolve;
[0084] (c) under continuous stirring at 1200 rpm, transfer (b) into (a) and stir for 20 minutes;
[0085] (d) Turn the mixture to 35°C, stir continuously at 1200 rpm for 15 minutes, add baricitinib and stir evenly, then add sodium hydroxide solution to adjust the pH value to 6.0-7.0;
[0086] (e) filling to obtain baricitinib emulsifiable cream.
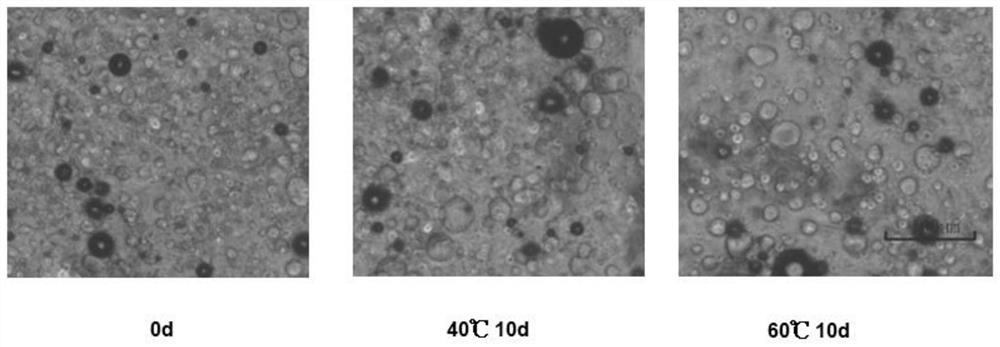
[0087] The morphology of...
Embodiment 3
[0089] Recipe for Cream 3
[0090] The composition of the preparation prescription is the content of components in every 100 grams of cream:
[0091] Baricitinib 250 mg;
[0092] Polyethylene glycol-7 stearate (Tefose 63) 14.5 grams;
[0093] 6 grams of liquid paraffin;
[0094] Carbopol (Carbopol 980) 0.02 grams;
[0095] Phenoxyethanol 0.45 g;
[0096] 0.005 grams of disodium edetate;
[0097] Appropriate amount of sodium hydroxide;
[0098] Purified water was added to make up to 100 g.
[0099] making process:
[0100] (a) Polyethylene glycol-7 stearate and liquid paraffin are melted at 60°C;
[0101] (b) Swell the carbomer with water for 6 hours, heat to 60°C, add phenoxyethanol and disodium edetate to dissolve;
[0102] (c) under continuous stirring at 1200 rpm, transfer (b) into (a) and stir for 20 minutes;
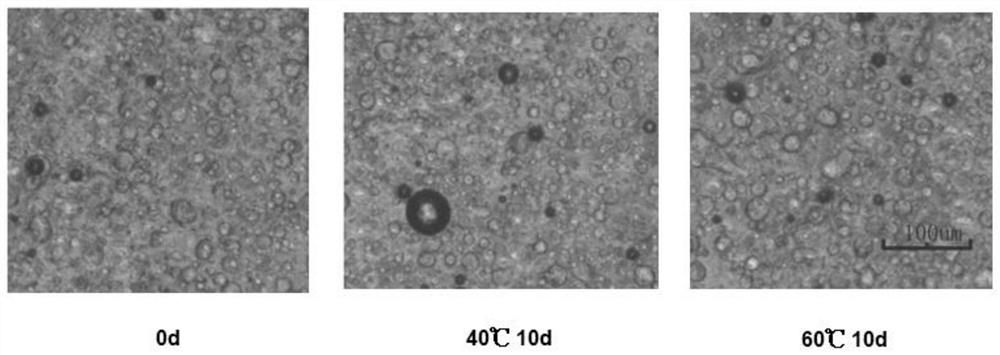
[0103] (d) Turn the mixture to 35°C, stir continuously at 1200 rpm for 15 minutes, add baricitinib and stir evenly, add sodium hydroxide solution to adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com