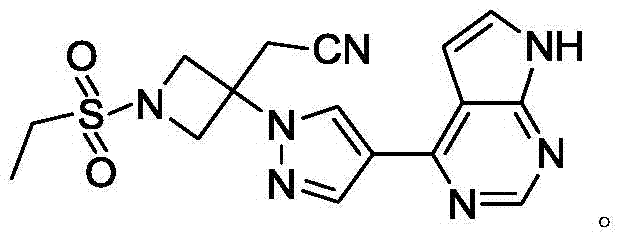
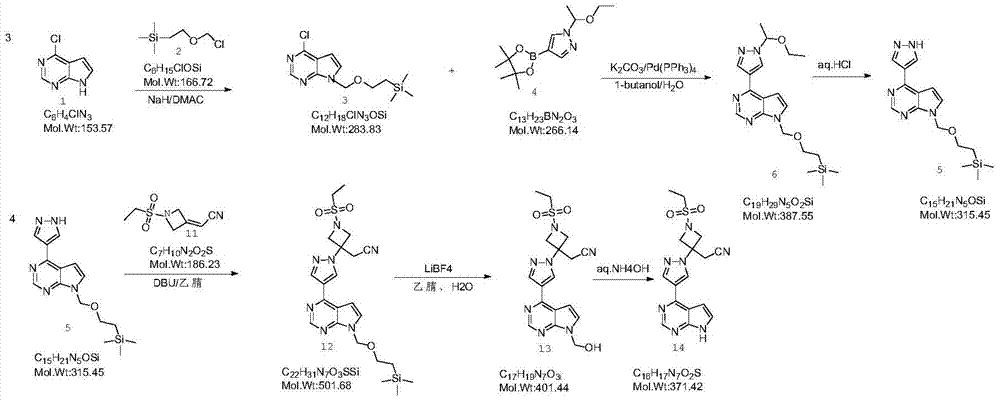
Baricitinib intermediate and preparation method thereof and method for preparing baricitinib from the intermediate
A baricitinib and intermediate technology, applied in the field of small molecule drug preparation, can solve the problems of limited industrial production of compounds, complex by-products, numerous reaction routes, etc., and achieves good market application prospects, high product purity, and simple process steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
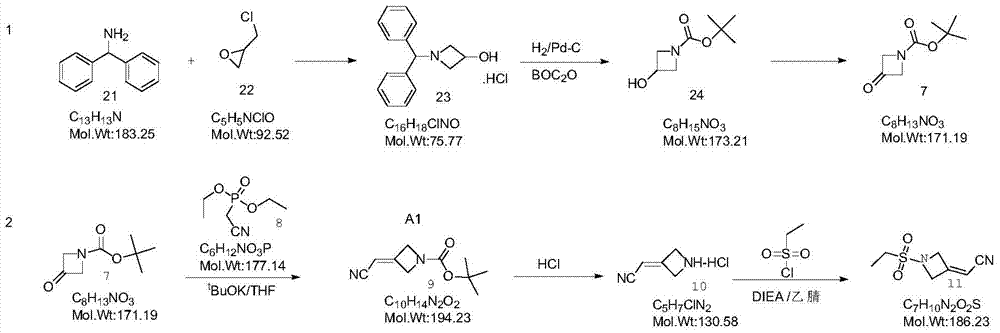
[0066] (1) Preparation of compound 1
[0067] The preparation of compound 1 is an existing technology, and the method disclosed in the patent application with the international publication number WO 2009 / 114512Al can be referred to. In this example, compound 1 was directly purchased from the company.
[0068] (2) Preparation of compound 2
[0069] The synthetic route is:
[0070]
preparation example 1-1
[0072] Sodium hydride (0.260g, 0.011mol) was dissolved in tetrahydrofuran solvent in a three-necked flask protected by nitrogen, cooled to 0°C, compound 7 (1100ml, 6.72mmol, 1.15equiv) was dissolved in tetrahydrofuran solvent, and slowly added to the previous solution . The mixture was raised to room temperature (25° C.) for 1 hour, then cooled to 0° C., and reacted for 1 hour. Compound 1 (1.0 g, 5.84 mmol) was dissolved in tetrahydrofuran solvent, the above mixture was added slowly, and stirred for 1 hour. Rise to room temperature (25°C) and react overnight (12h). Quenched with cold water, and the solvent tetrahydrofuran was removed by rotary evaporation. Extracted with ethyl acetate, the aqueous layer was separated, and the organic layer was washed with brine. Dry over anhydrous magnesium sulfate, filter, concentrate, and purify by column chromatography to obtain 0.939 g of white solid with a yield of 83%.
[0073] The data of the HNMR of target product compound 2 are as...
preparation example 1-2
[0077] The alkali catalyst sodium hydride was replaced by potassium tert-butoxide, and the other reaction conditions remained unchanged, as in Preparation 1-1, and the yield of the final target compound 2 was 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com