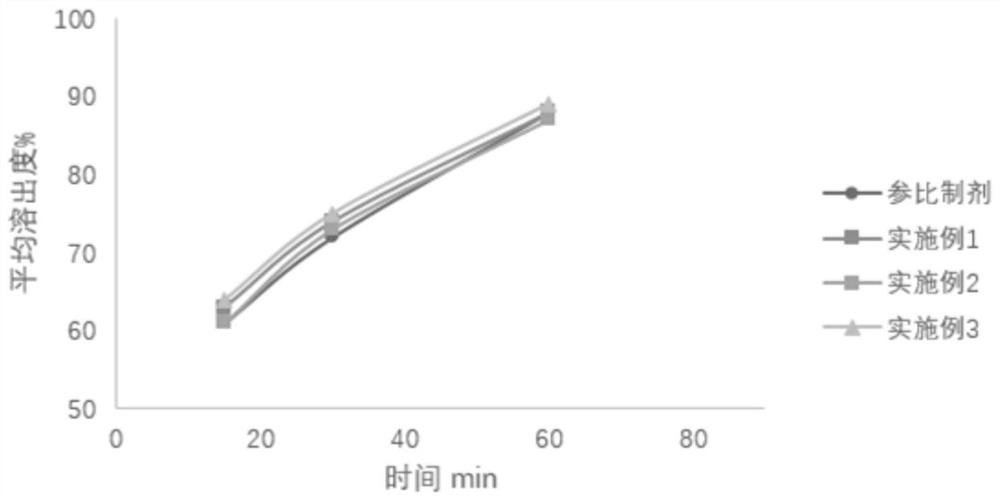
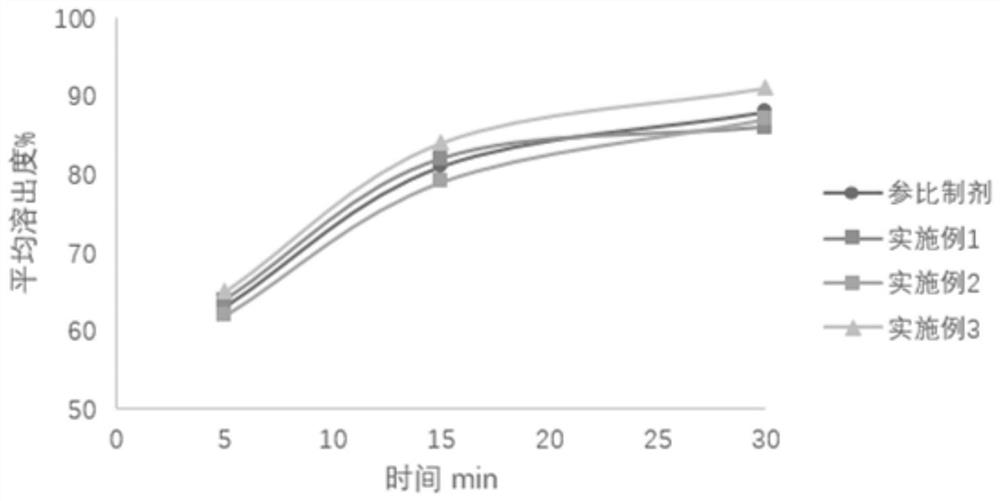
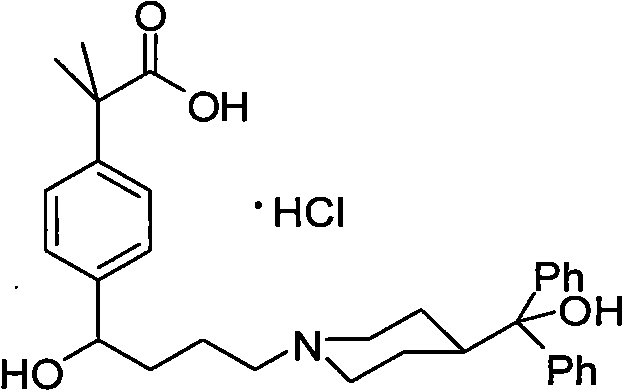
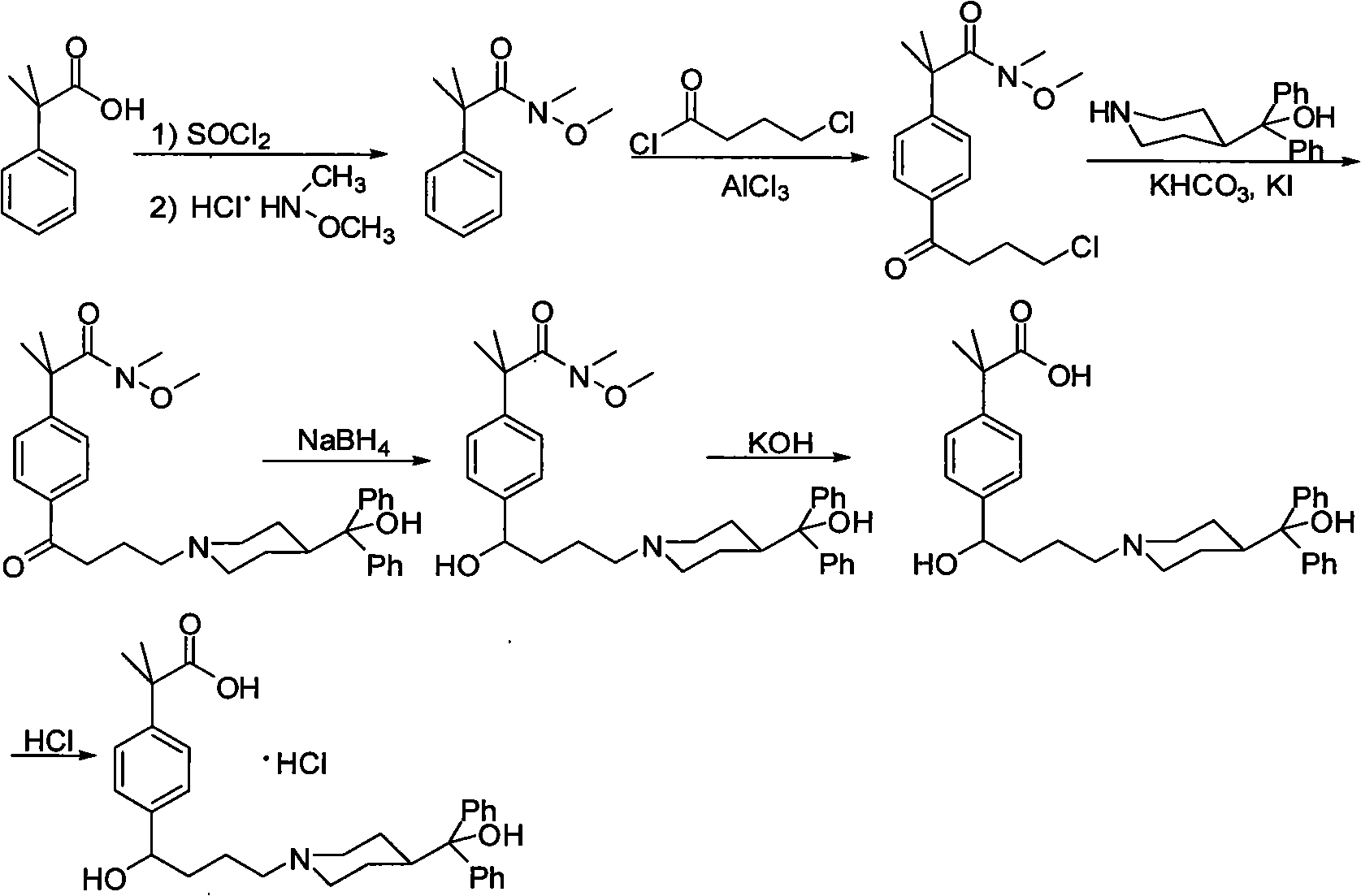
Patents
Literature
59 results about "Fexofenadine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
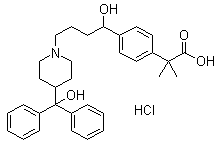
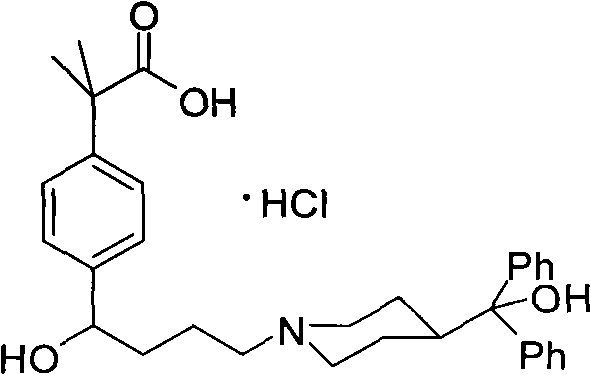
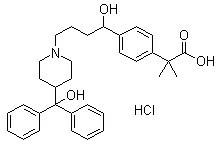
The hydrochloride salt form of fexofenadine, a carboxylated metabolic derivative of terfenadine and second generation, long-lasting selective histamine H1 receptor antagonist, with antihistaminic activity. Upon administration, fexofenadine competitively binds of peripheral H1-receptors in the gastrointestinal (GI) tract, blood vessels, and bronchial smooth muscle. This prevents binding of histamine to peripheral H1-receptors and prevents their activation. This prevents a histamine-mediated allergic reaction. Fexofenadine does not cross the blood-brain-barrier (BBB).
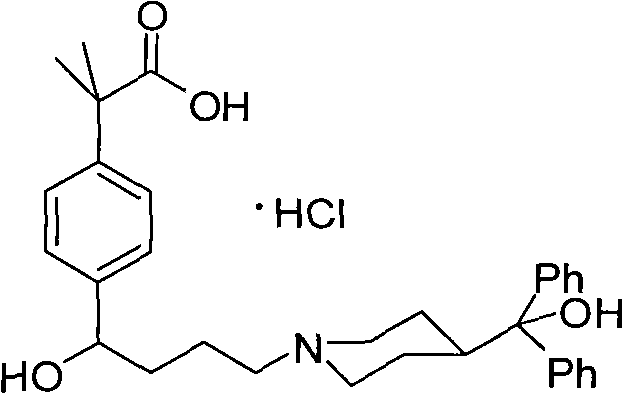
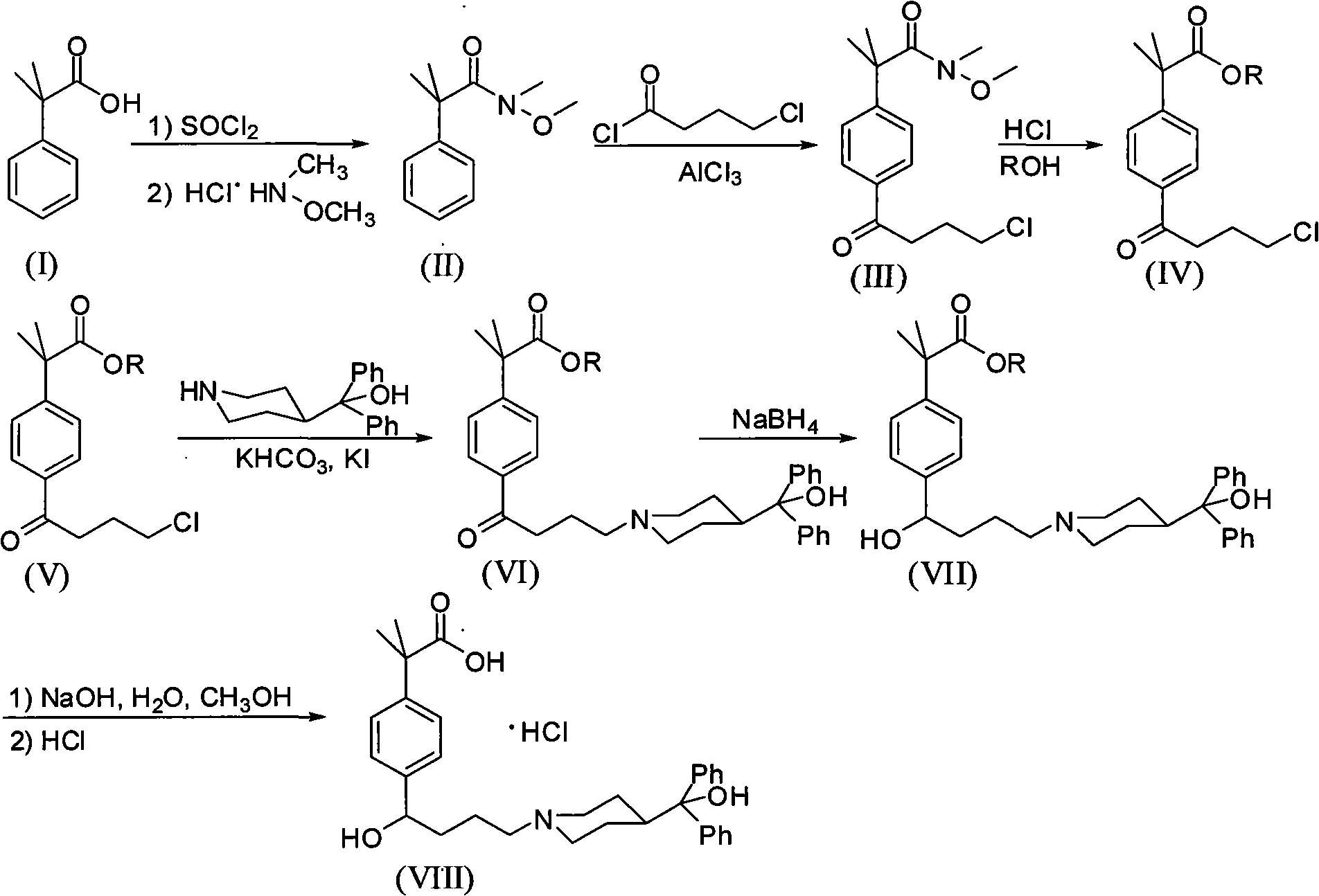
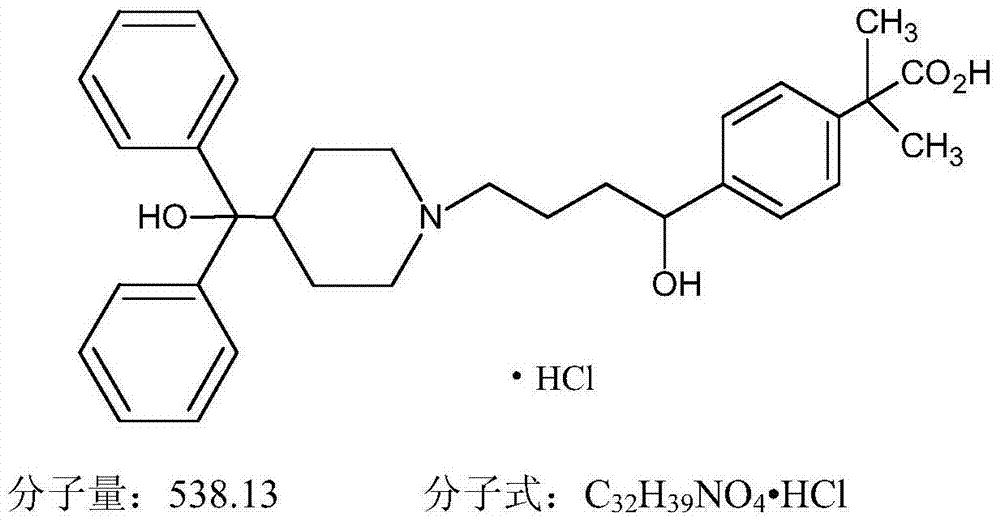
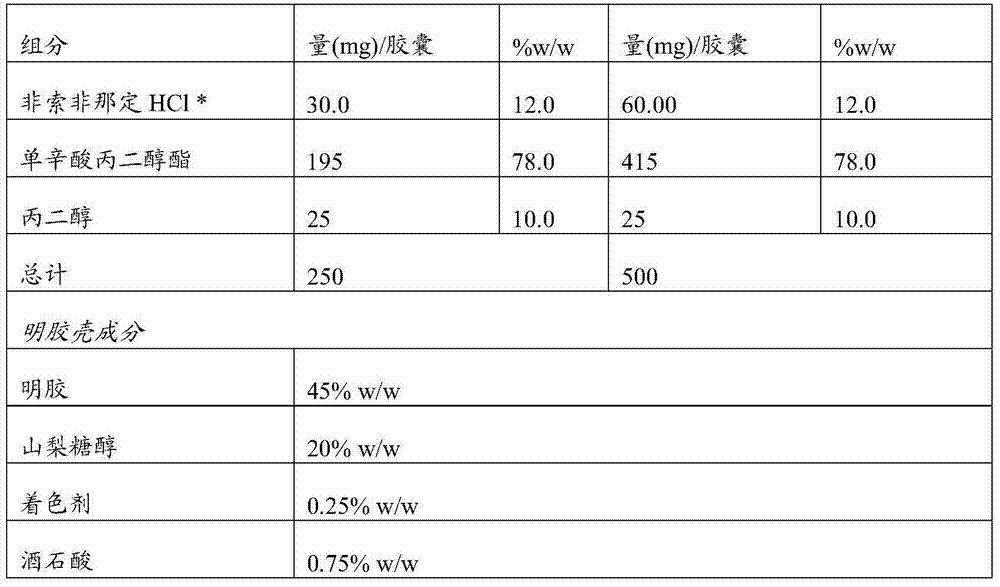
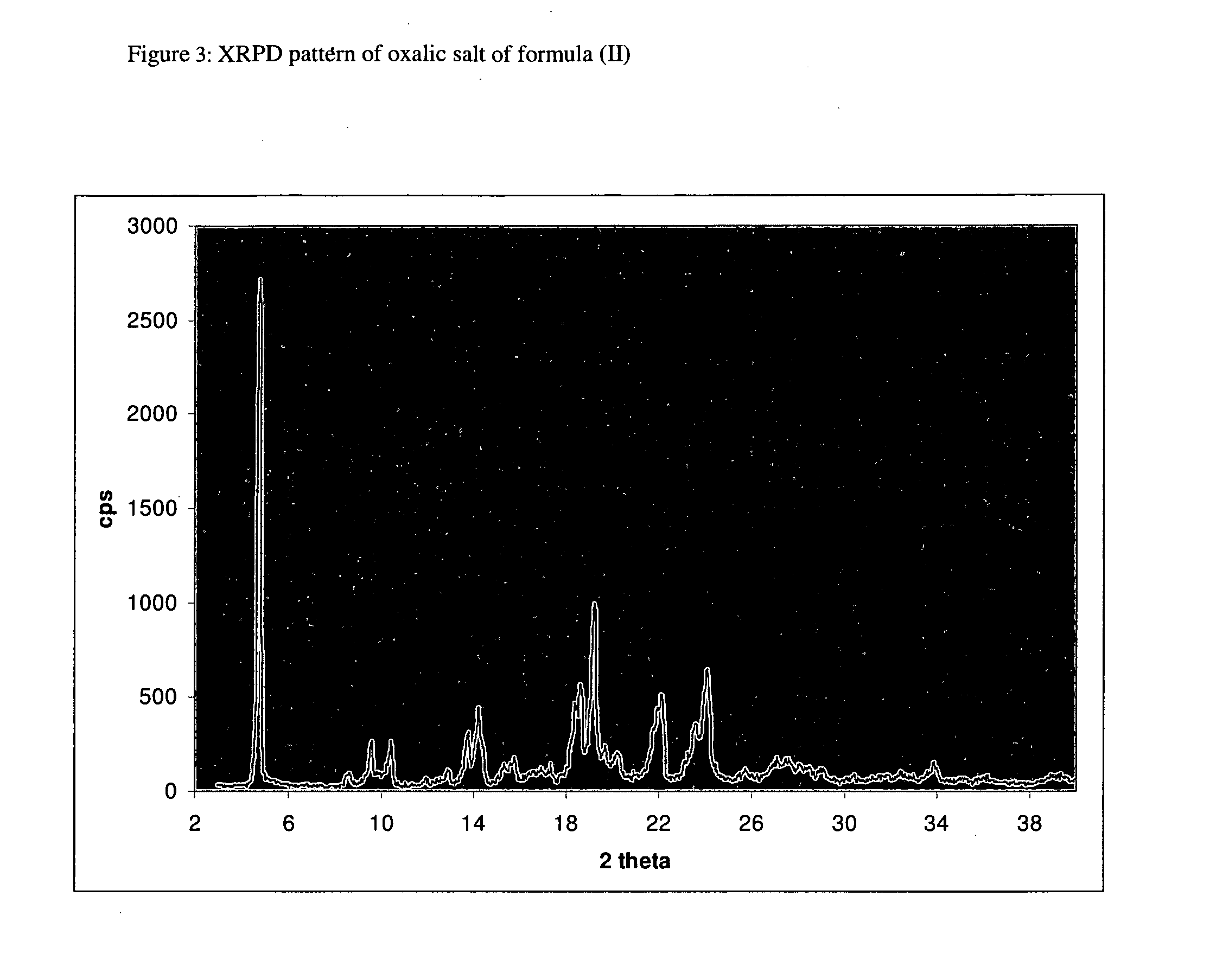
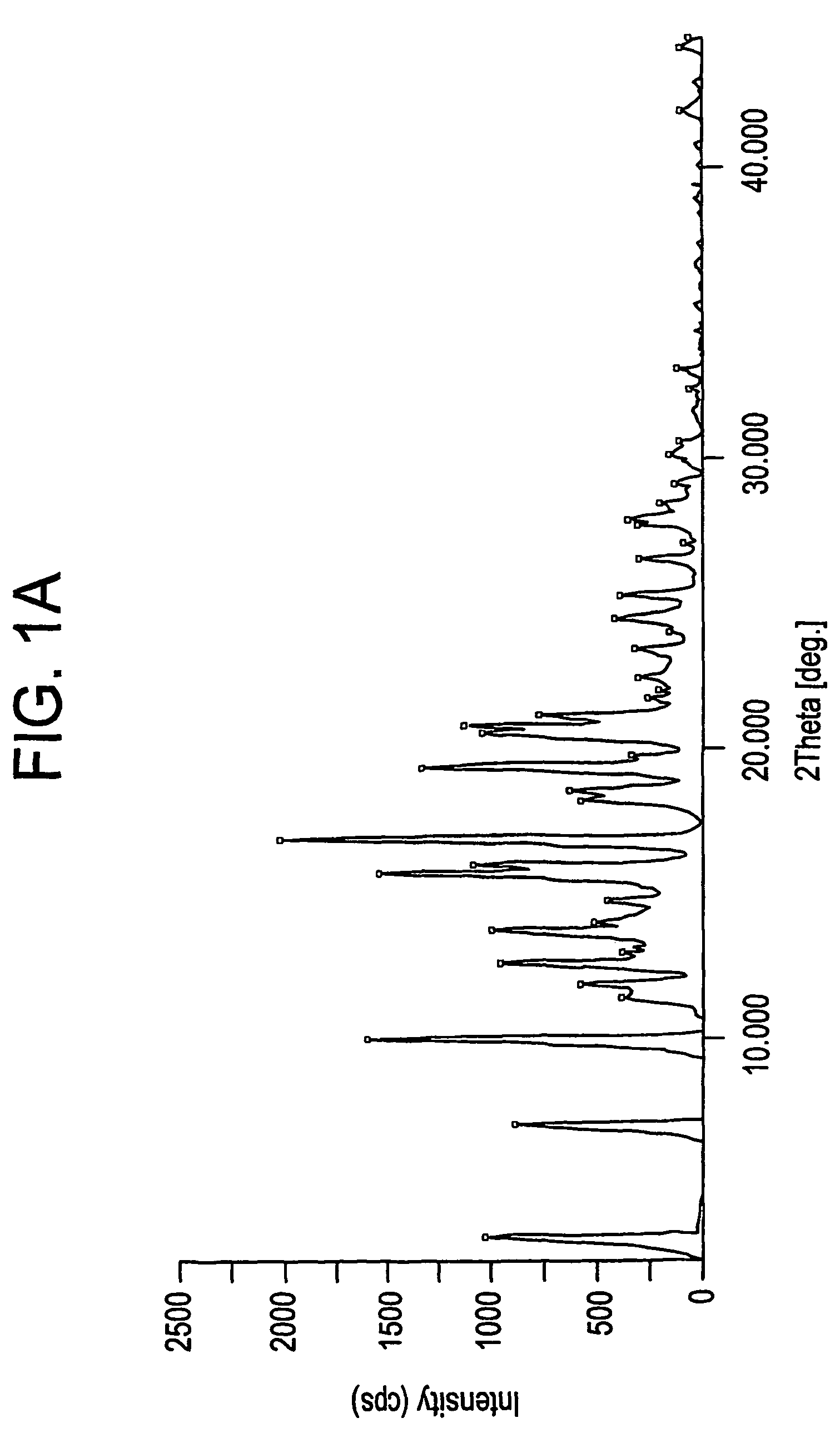
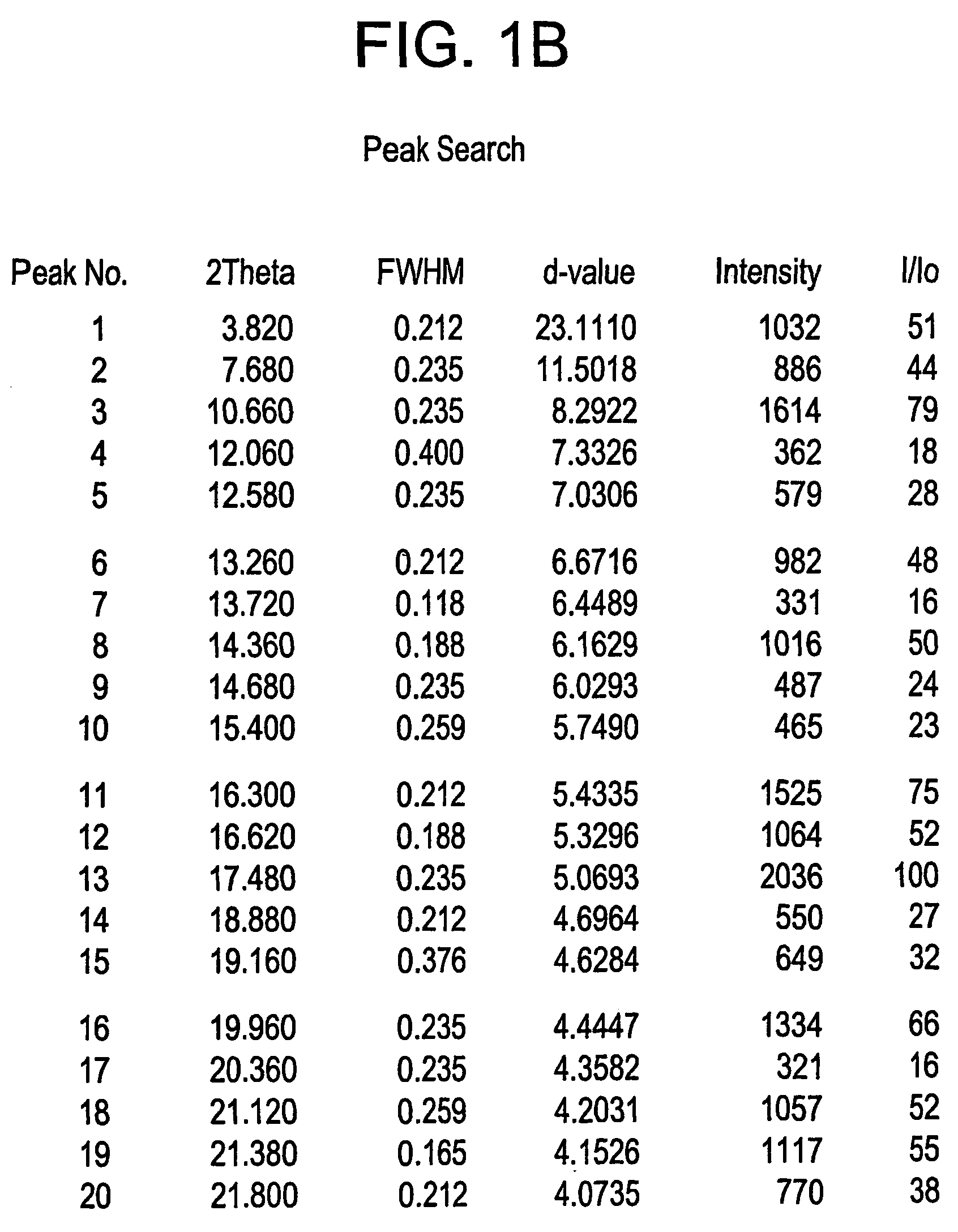
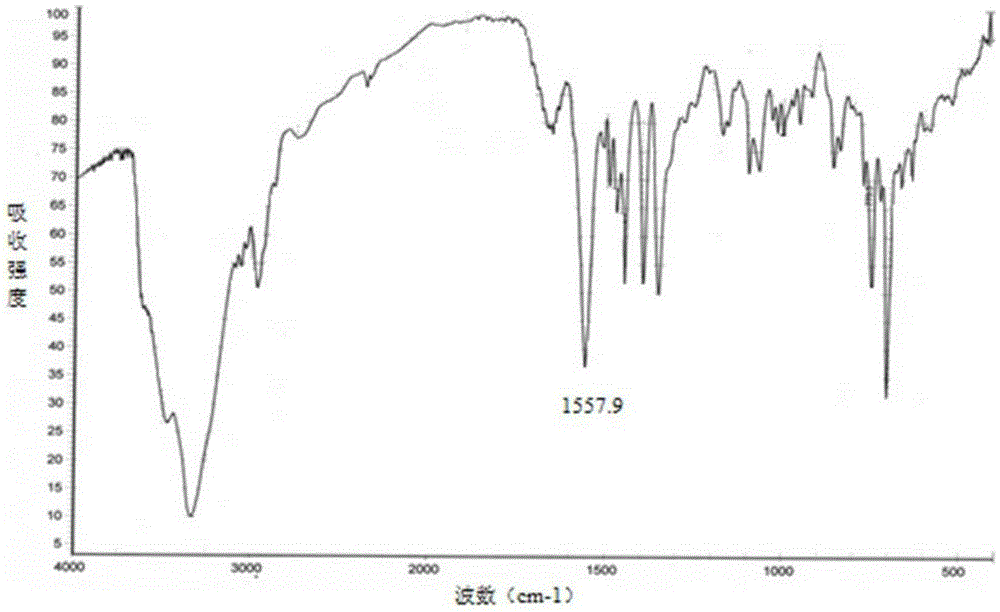
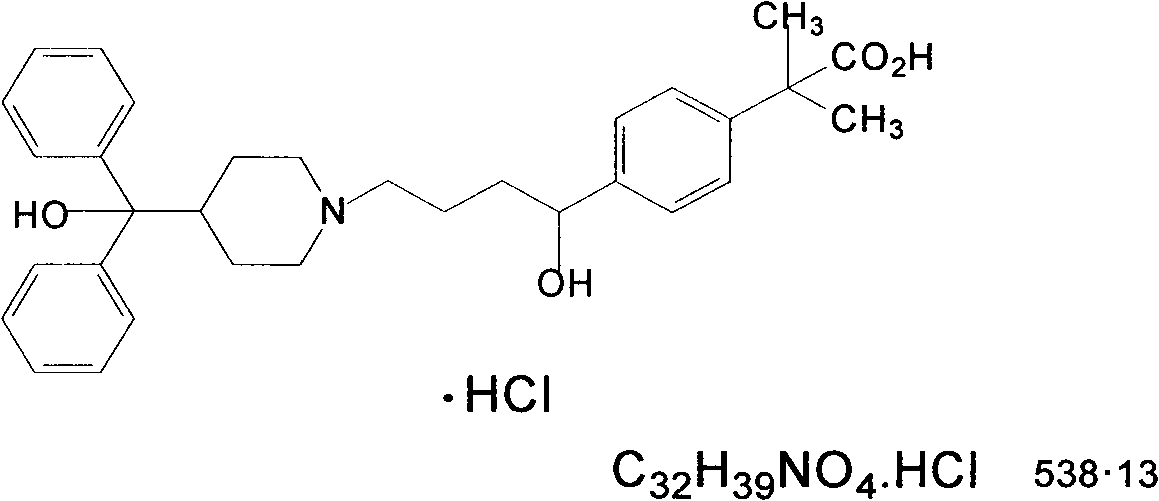
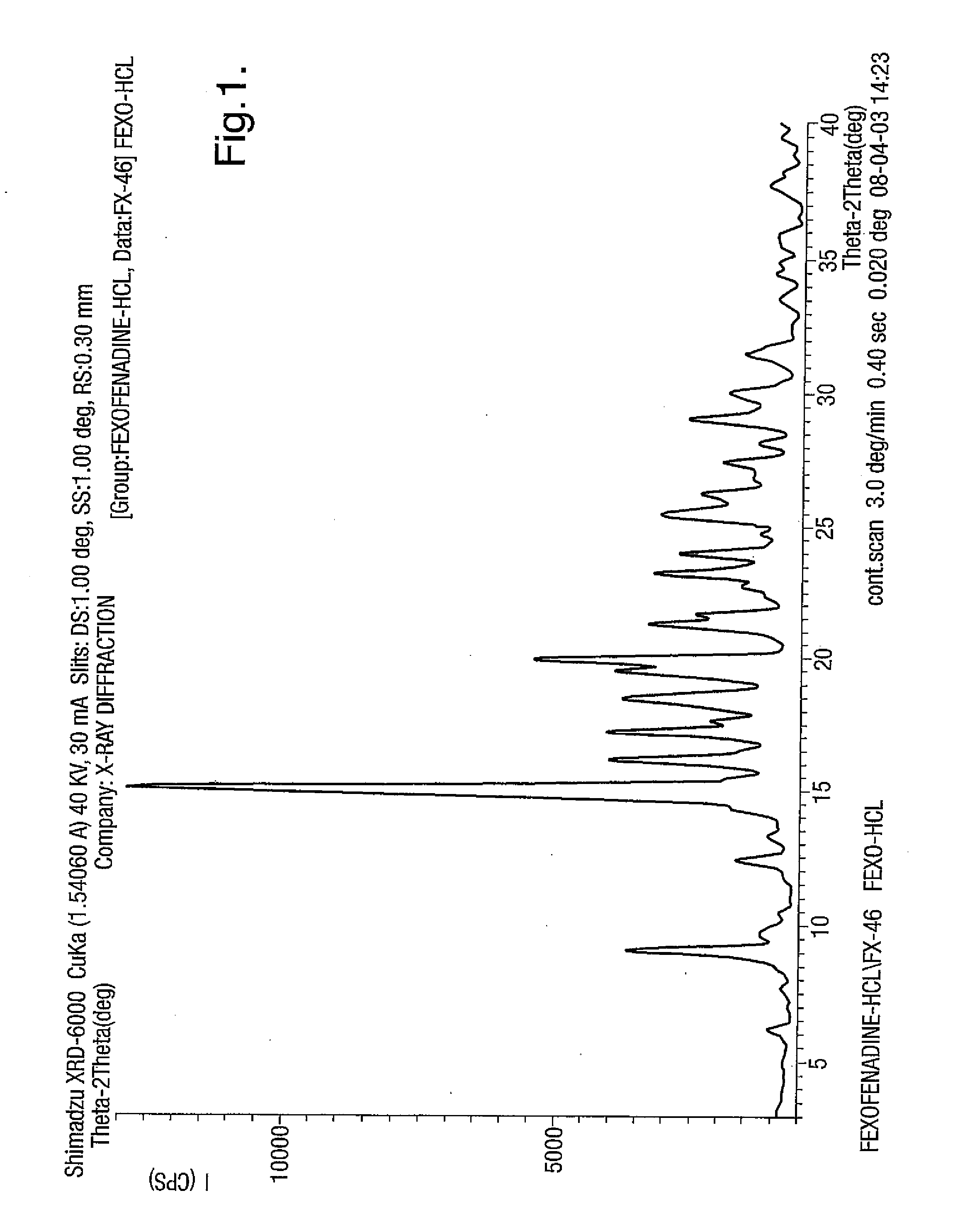
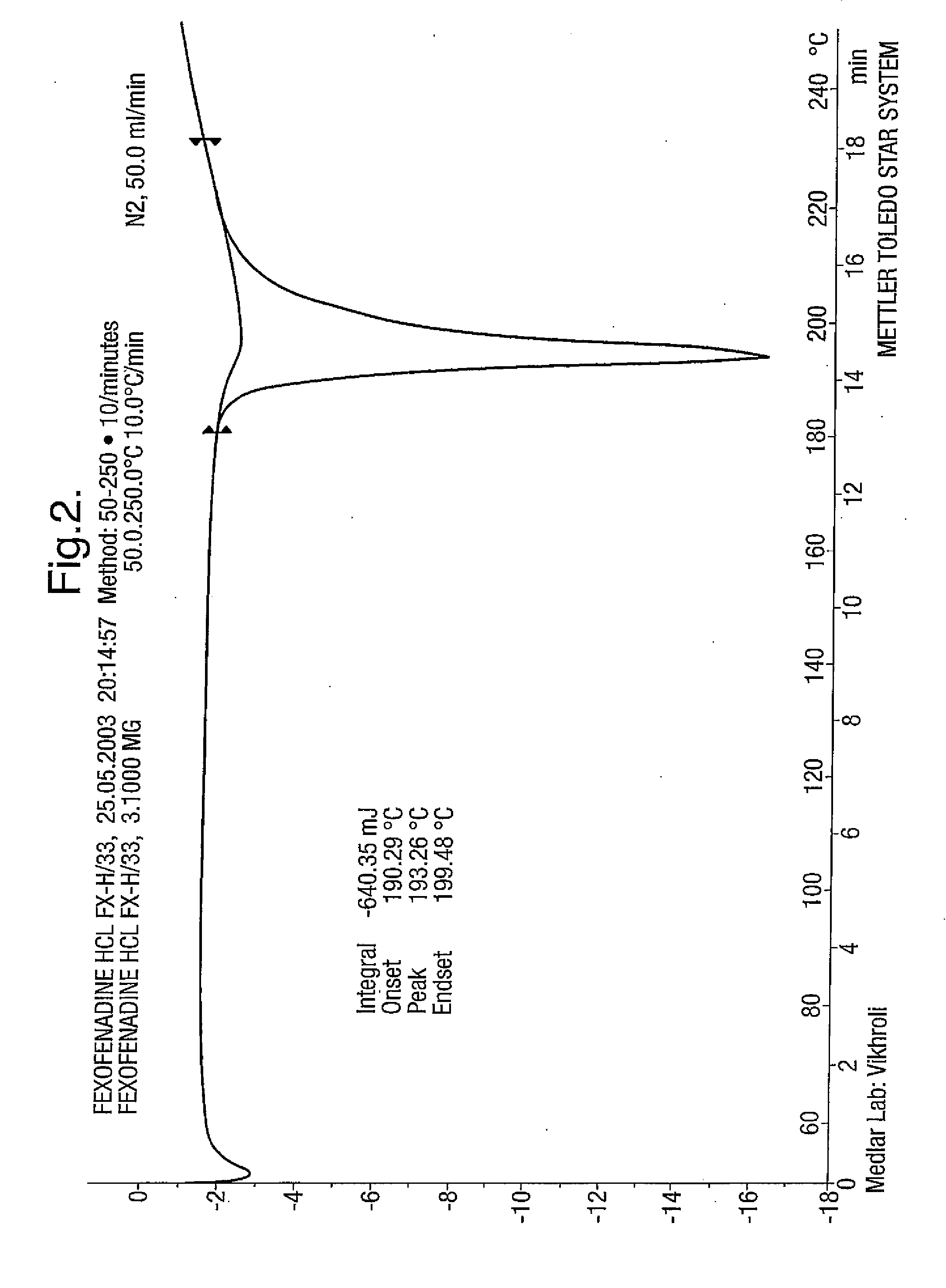
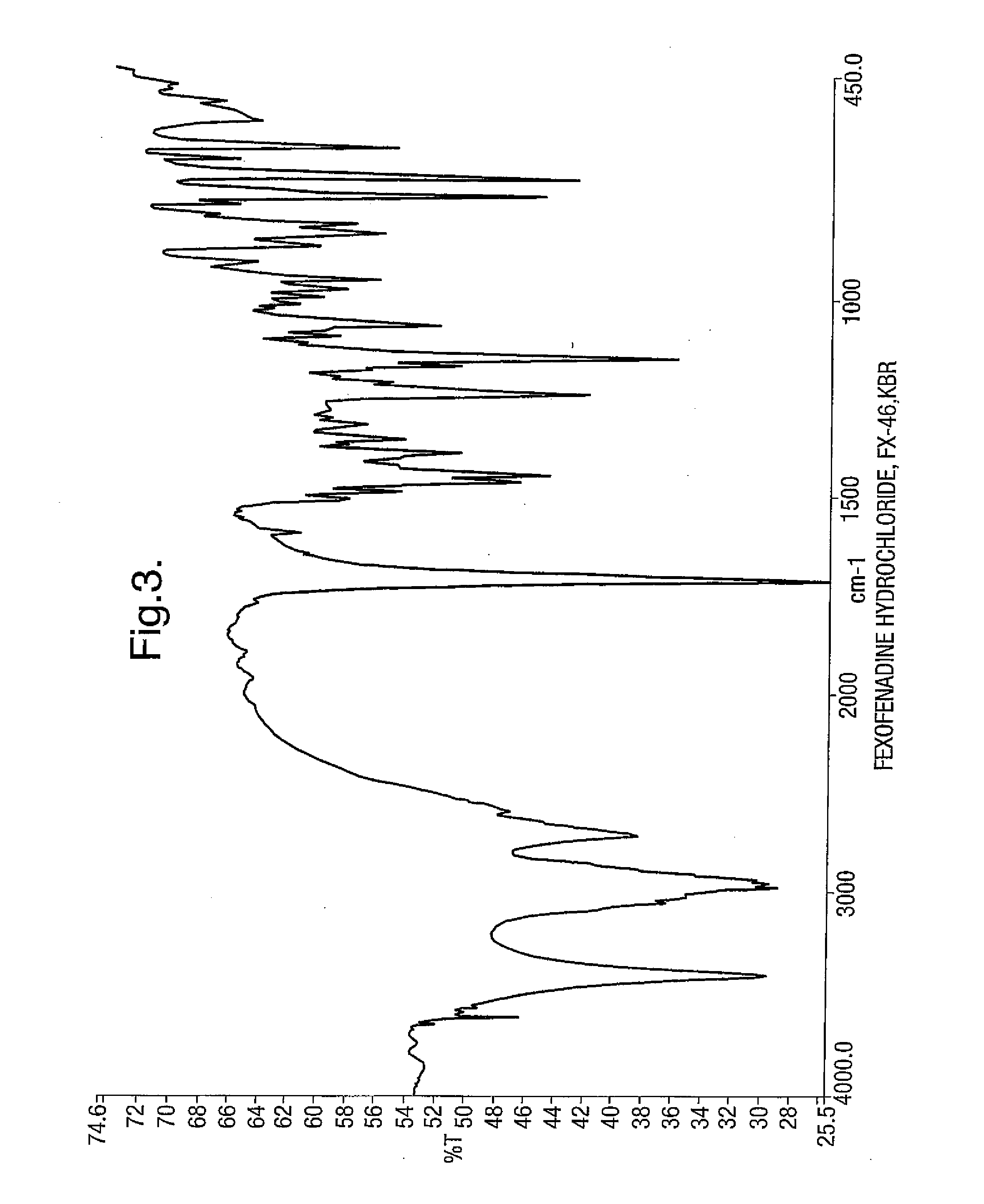
Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride
The present invention is related to novel polymorph of Fexofenadine and Fexofenadine hydrochloride of formula 1 and process of preparation thereof. The present invention is also directed to provide pure novel polymorphs of Fexofenadine and its hydrochloride by a simple process which is cost effective, commercially viable and environment friendly.
Owner:DR REDDYS LAB LTD
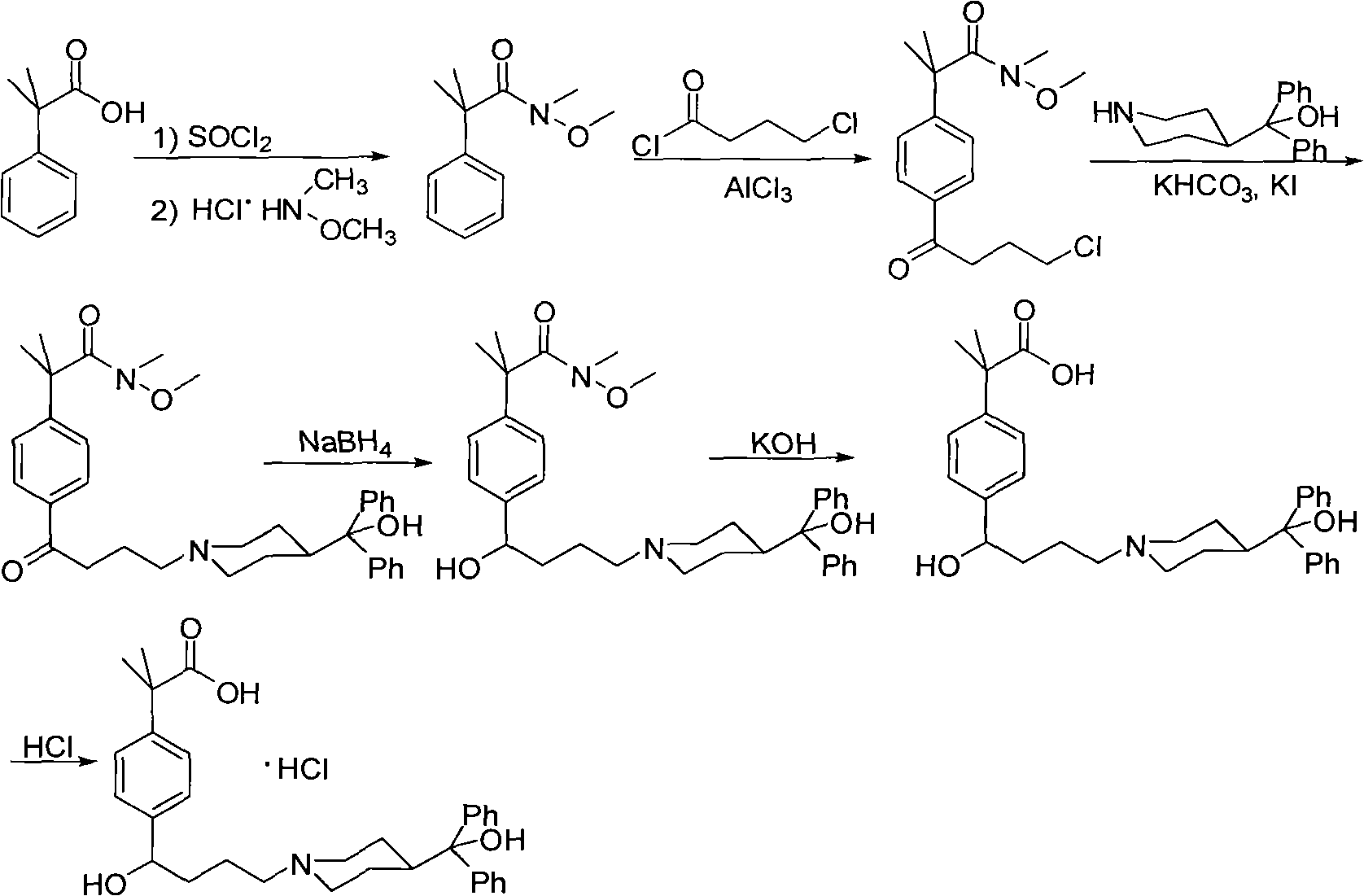
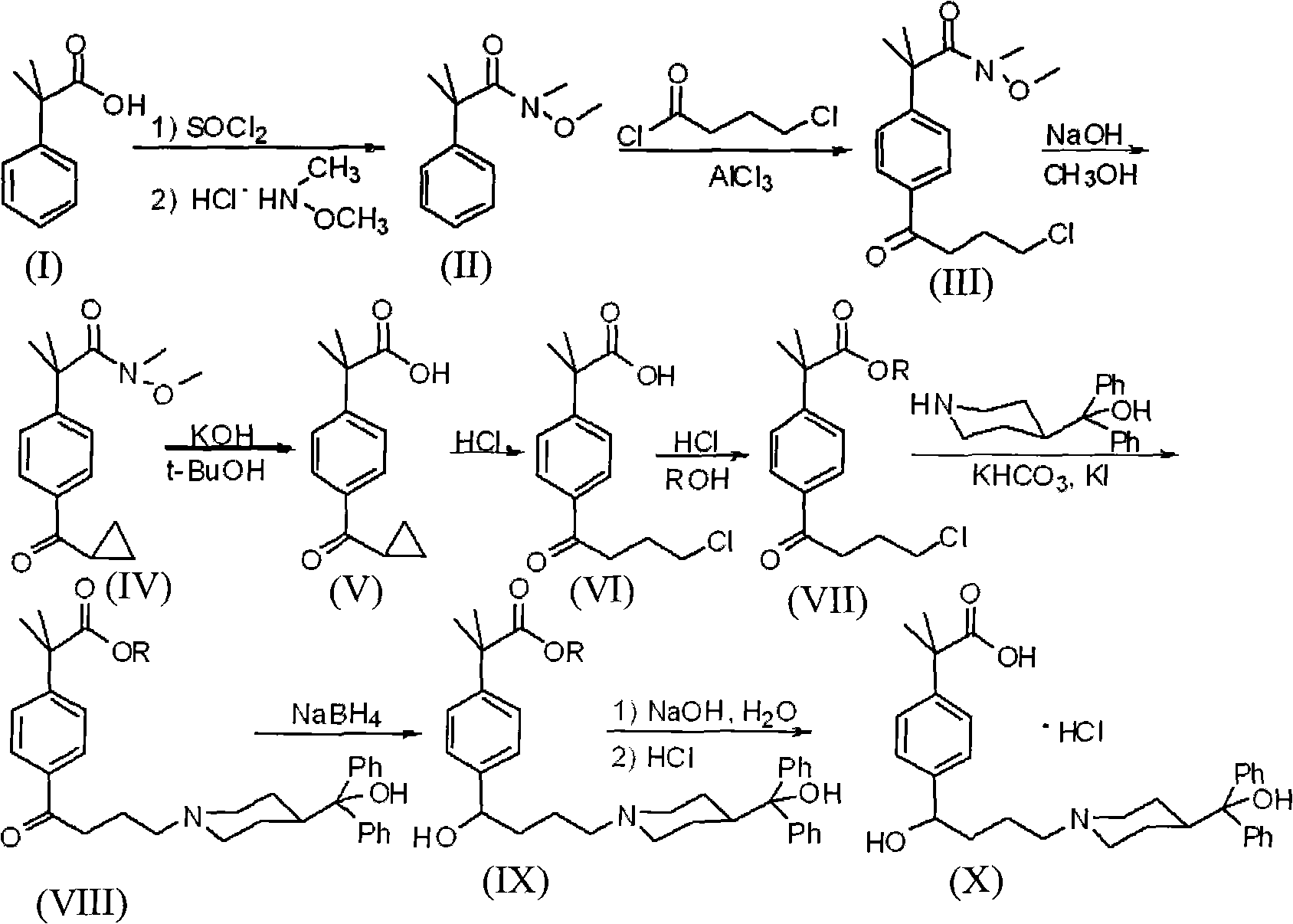
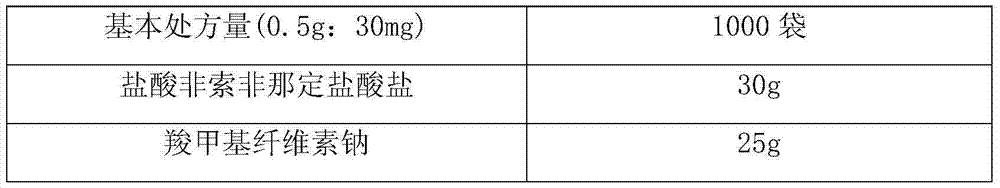
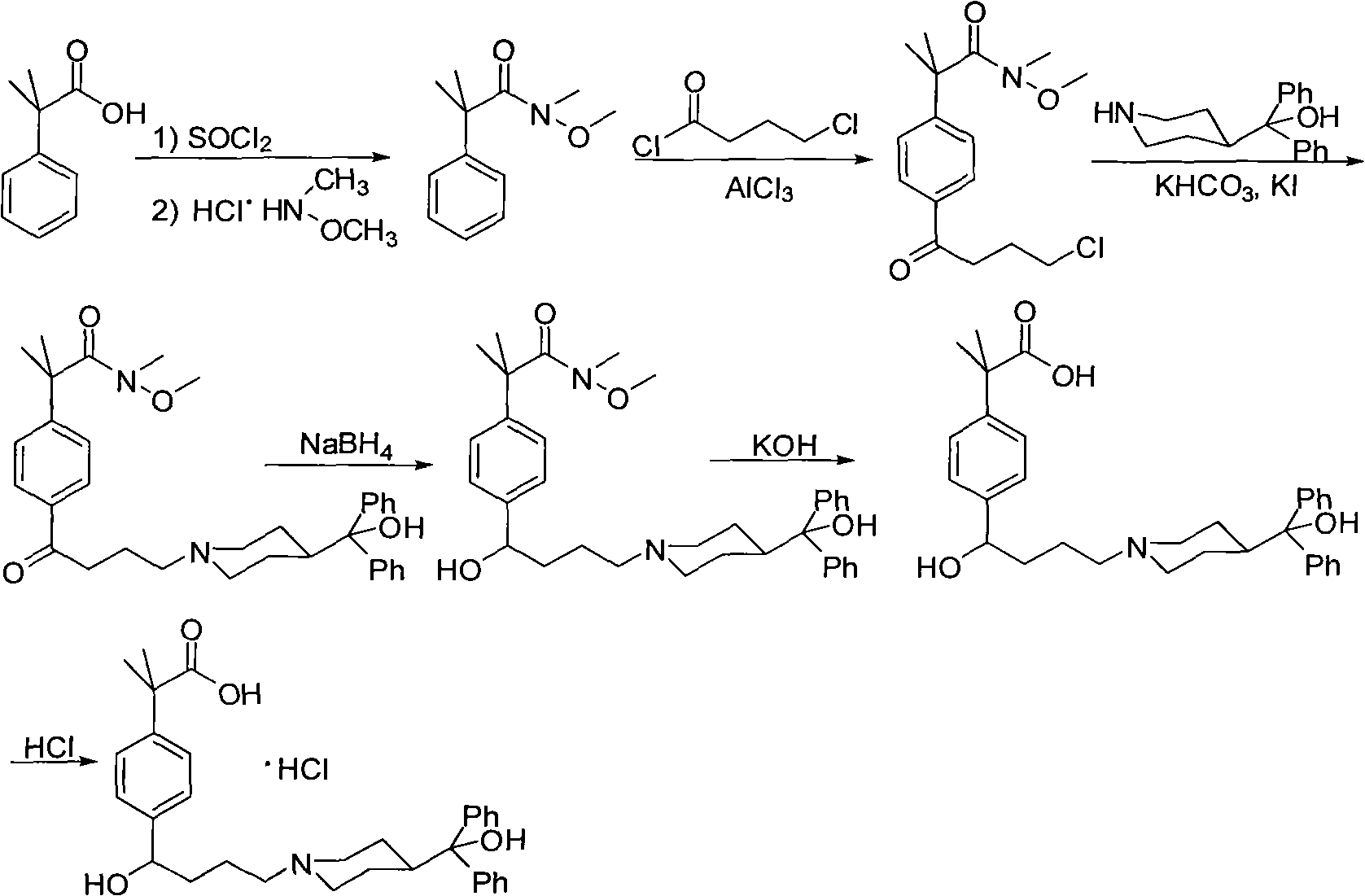
Synthetic method of fexofenadine hydrochloride
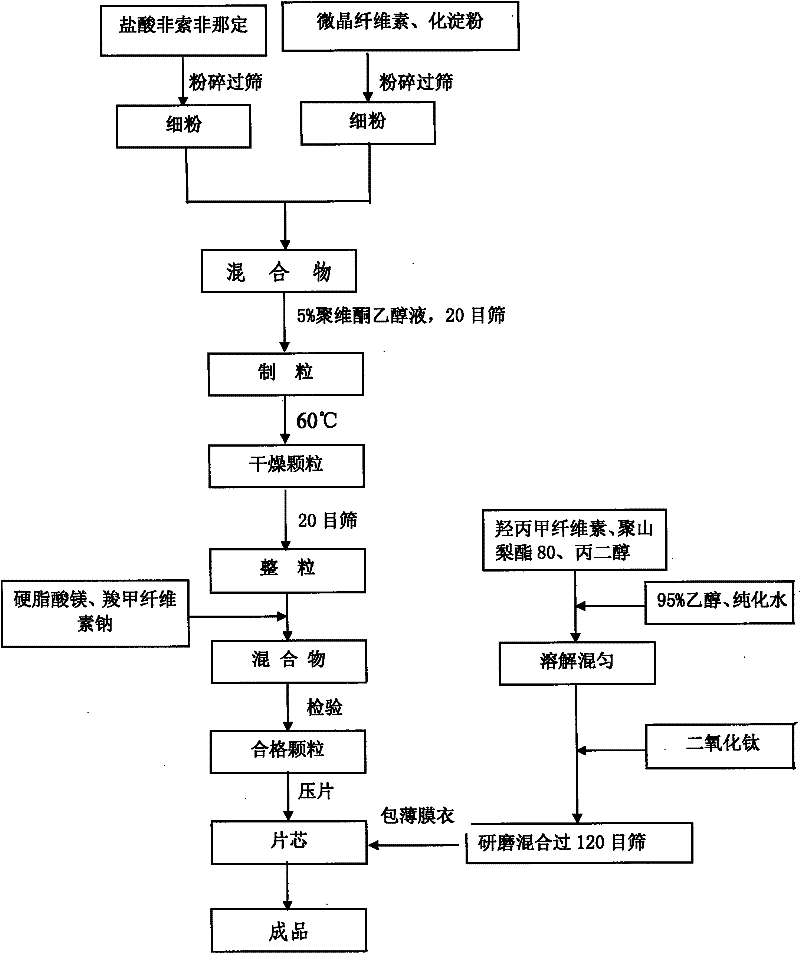
The invention discloses a synthetic method of fexofenadine hydrochloride. N-methyl-N-methoxyl-2-[4-(4-chlorine butyryl)]-2-methacrylamide which is prepared through the prior art is taken as the raw material, which is reacted in saturated hydrogen chloride absolute ethyl alcohol dissolvent, then 2-[4-(4-chlorine butyryl)]-2-propyl methacrylate is obtained, and finally the object product is preparedthrough a series of reactions. The synthetic method of fexofenadine hydrochloride has the advantages of having high yield, no meta-isomer and amide impurities existence, little pollution, simple processing procedure, low production cost and being suitable for the industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
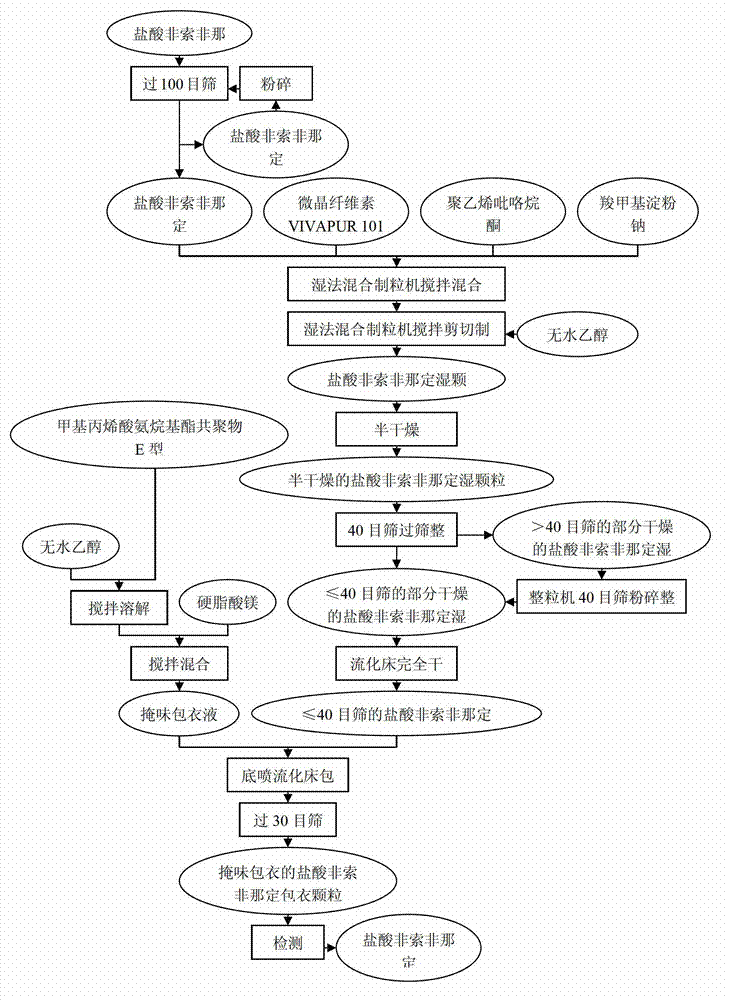
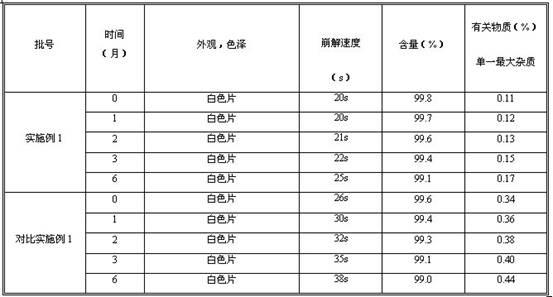
Fexofenadine hydrochloride orally disintegrating tablet and preparation method thereof
InactiveCN101756979APromote absorptionFast absorptionPill deliveryImmunological disordersSocial benefitsPhysical well being
The invention provides a preparation method of fexofenadine hydrochloride orally disintegrating tablets. Based on the advantages of the orally disintegrating tablet preparation formulation, the product not only overcomes the defects of poor disintegration, slow effect, low bioavailability and the like of the preparation formulations on sale in foreign countries, but also fills a gap in the domestic market, thus adapting to the demand of development of market situation. The medicine has important significance in keeping good health of the people and creating economical and social benefits. The product has simple and easy technique and easily obtained raw and auxiliary materials, is suitable for industrial production, and has good application prospect.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
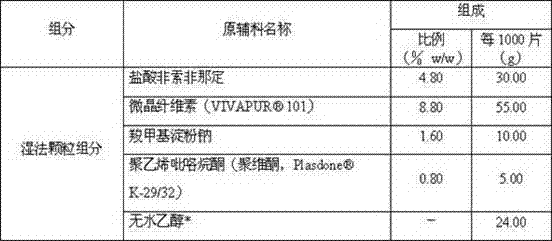
Method for preparing fexofenadine hydrochloride orally disintegrating tablet
ActiveCN102885791AExtended storage timeShort disintegration timePharmaceutical non-active ingredientsPill deliveryAlcoholOrally disintegrating tablet
The invention provides a method for preparing a fexofenadine hydrochloride orally disintegrating tablet. The method comprises the following steps of: (1) providing component substances including fexofenadine hydrochloride, microcrystalline cellulose, sodium carboxymethyl starch, polyvidone and absolute ethyl alcohol in the mass ratio of 300:550:100:50:240; (2) adding the fexofenadine hydrochloride, microcrystalline cellulose, sodium carboxymethyl starch and polyvidone in the component substances, adding a mixture into a wet mixing pelletizer, and starting shearing and mixing, wherein the rotating speed of a stirring paddle is 504 revolutions per minute, the rotating speed of a pelletizing knife is 1,500 revolutions per minute, and the mixing time is 10 minutes; (3) adding absolute ethyl alcohol into a powdery mixture, and mixing continually under the condition that the rotating speed of a stirring paddle wheel is 756 revolutions per minute, the rotating speed of the pelletizing knife is 3,000 revolutions per minute to obtain wet particles; and (4) drying the obtained wet particles obtained in the step (3) in a half way.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
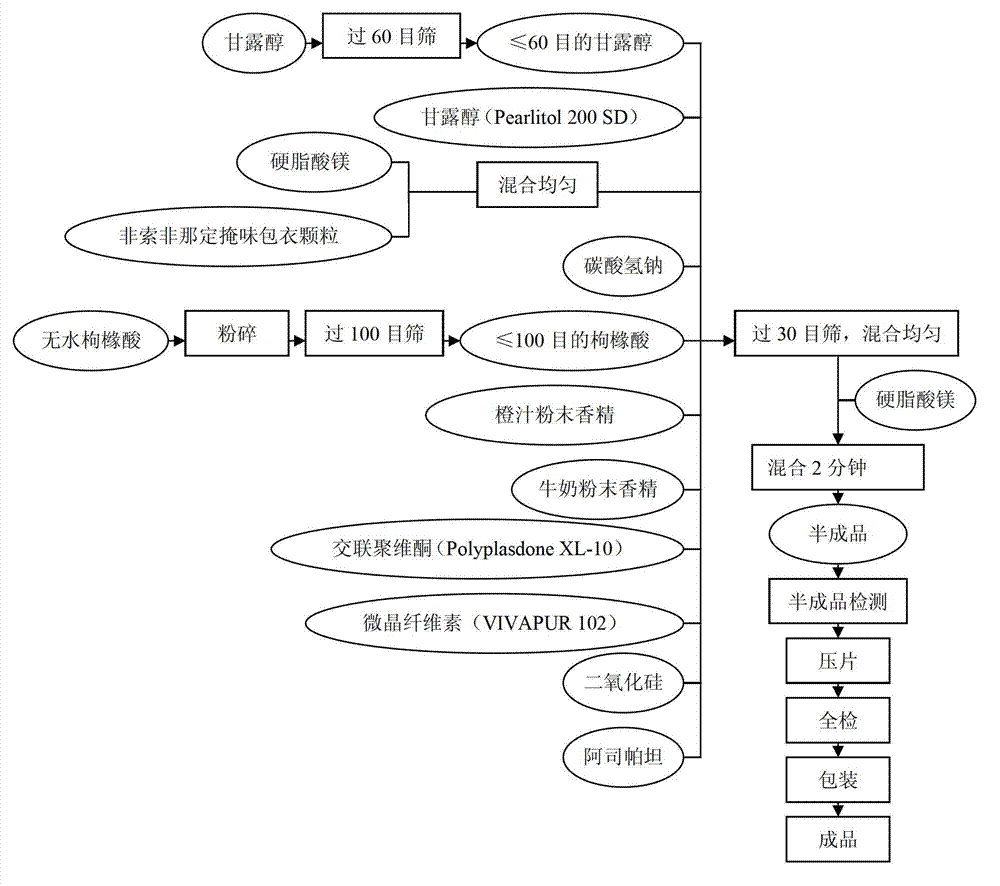
Pharmaceutical composition with fexofenadine hydrochloride and preparation method of pharmaceutical composition
ActiveCN103610645AImprove bioavailabilityGreat tastePowder deliveryRespiratory disorderDispersityBioavailability
The invention relates to a pharmaceutical composition with fexofenadine hydrochloride. The composition is composed of the following components: 1-12% of fexofenadine hydrochloride as an active ingredient, 1-10% of suspending aid, 5-85% of filler, 1-10% of sweetening agent, 0.01-10% of essence and a conventional amount of flow aid, wherein the composition exists in a form of a dry suspension. The fexofenadine hydrochloride suspension provided by the invention has the advantages of high dispersity, uniformity in distribution, rapidness in absorption, high bioavailability, good taste, stability and the like.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
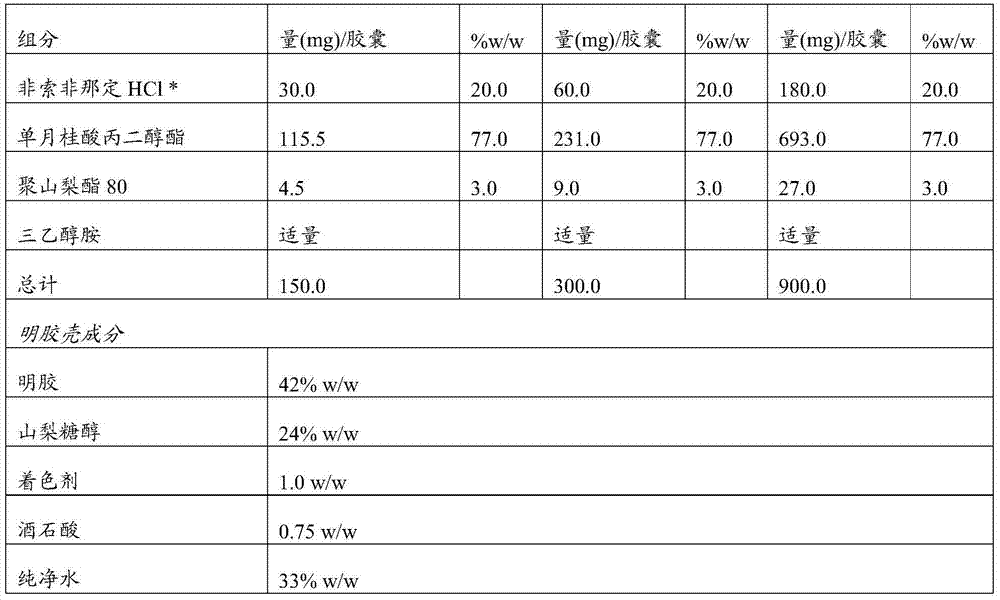
Pharmaceutical composition comprising fexofenadine
InactiveUS20140073670A1Improve drug bioavailabilityPalatable and stableBiocidePharmaceutical non-active ingredientsPharmaceutical formulationAllergic reaction
The present invention relates to a pharmaceutical formulation of fexofenadine hydrochloride in a solvent system suitable as a liquid fill composition.In another aspect, the invention also relates to a process for the preparation of the pharmaceutical formulation and the use of the composition for the treatment of allergic reactions in a patient.
Owner:AVENTISUB LLC
Fexofenadine hydrochloride dropping pill and its preparing method
InactiveCN1813730ARapid dissolutionHigh dissolution ratePill deliveryImmunological disordersFexofenadine HydrochlorideChronic idiopathic urticaria
The present invention discloses a medicine fexofenadine hydrochloride dripping pills preparation for effectively curing seasonal allergic rhinitis and chronic idiopathic urticaria and its preparation process. It is made up by using fexofenadine hydrochloride and matrix for making dripping pills.
Owner:陈茜
Pharmaceutical composition comprising fexofenadine
InactiveCN103687592AEasy to understandOrganic active ingredientsPharmaceutical non-active ingredientsPharmaceutical formulationAllergic reaction
Owner:AVENTIS PHARMA INC
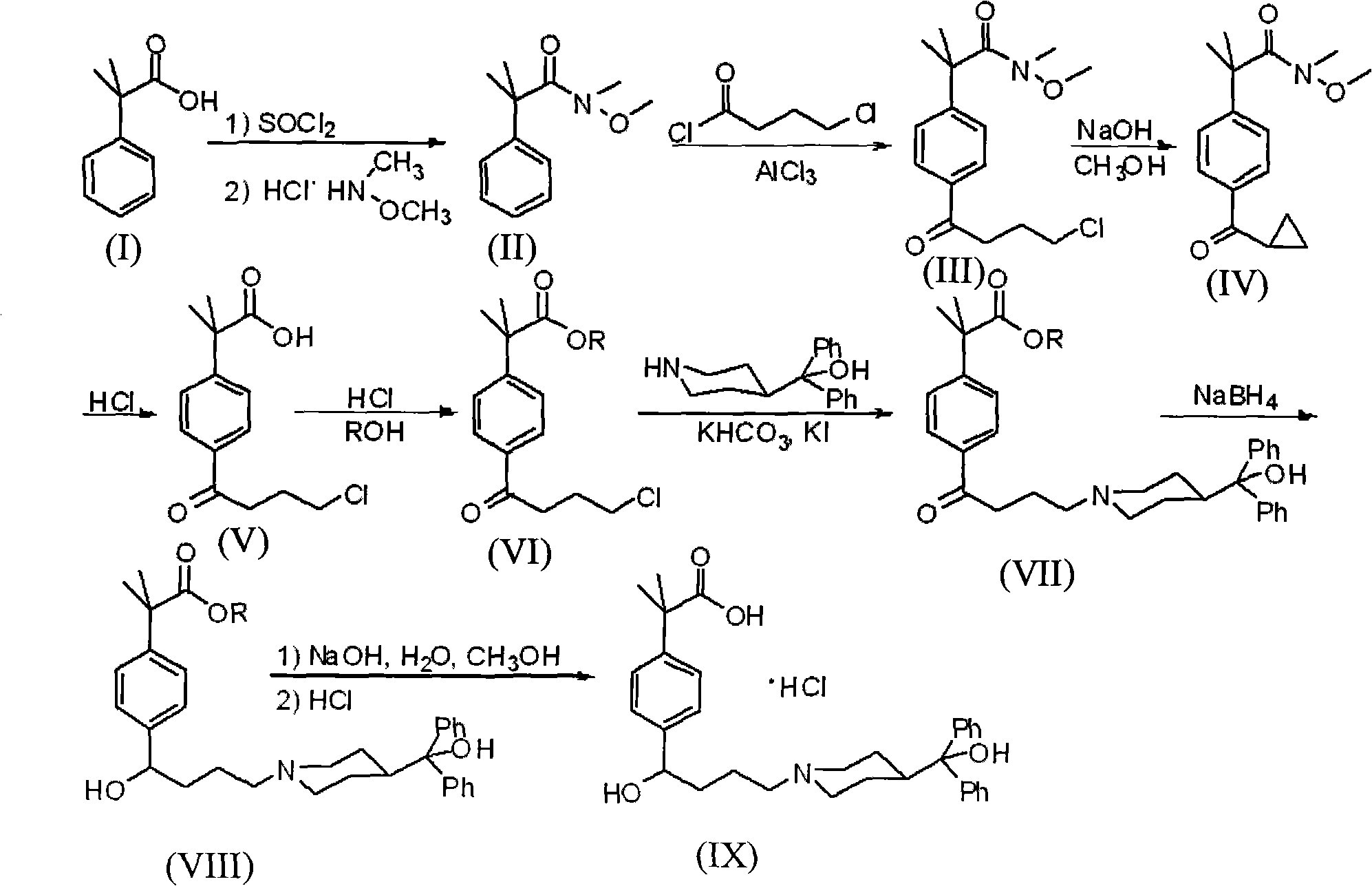
Preparation method of an antiallergic agent fexofenadine hydrochloride
InactiveCN101585805AHigh yieldReduce pollutionOrganic chemistryImmunological disordersMethacrylate2-methylpropanoic acid
The invention discloses a preparation method of an antiallergic agent fexofenadine hydrochloride, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting for 10-30h at 20-50 DEG C, conventionally processing to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an inorganic acid, reacting for 20-30h at 60 DEG C, conventionally processing and crystallizing by ethanol to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid in the alcohol solvent in which HCl gas is infused, conventionally processing to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, and finally obtaining the target product of the invention through routine techniques. The invention has the advantages of high yield, freeness of meta-isomers and amide impurities, little pollution and applicable industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Fexofenadine hydrochloride oral disintegrating drug composition
InactiveCN102512389AImprove compliancePrescription Analysis Angle StabilityPill deliveryPharmaceutical non-active ingredientsBioavailabilityPharmaceutical Substances
The present invention provides a fexofenadine hydrochloride oral disintegrating drug composition. The fexofenadine hydrochloride oral disintegrating drug composition has characteristics of good stability, high bioavailability, and cost reducing. With the fexofenadine hydrochloride oral disintegrating drug composition of the present invention, the industrialization is achieved, the good clinical application is provided, and the obvious advantage is provided.
Owner:JIANGSU GAOSHIDA ELECTRIC TOOL CO LTD
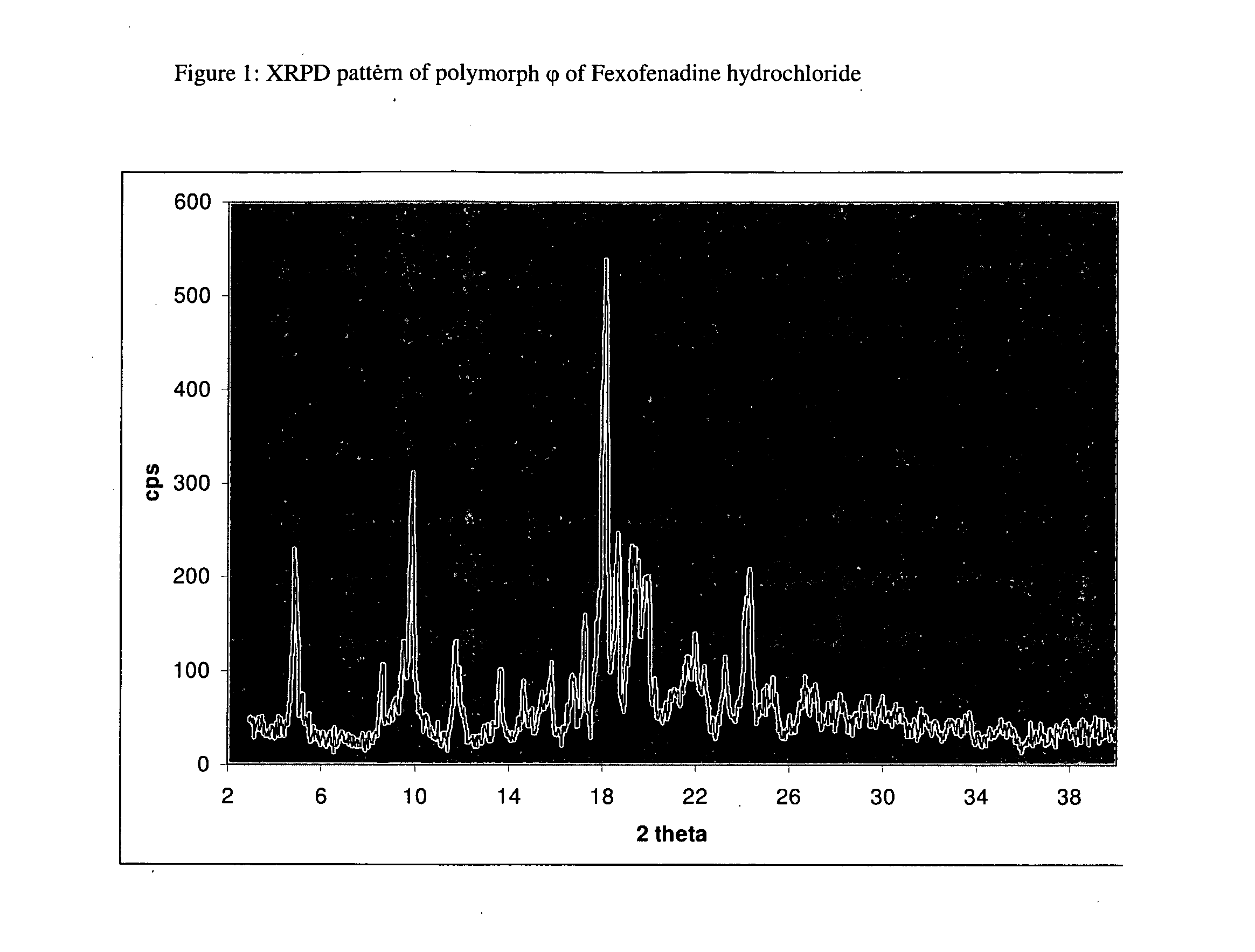
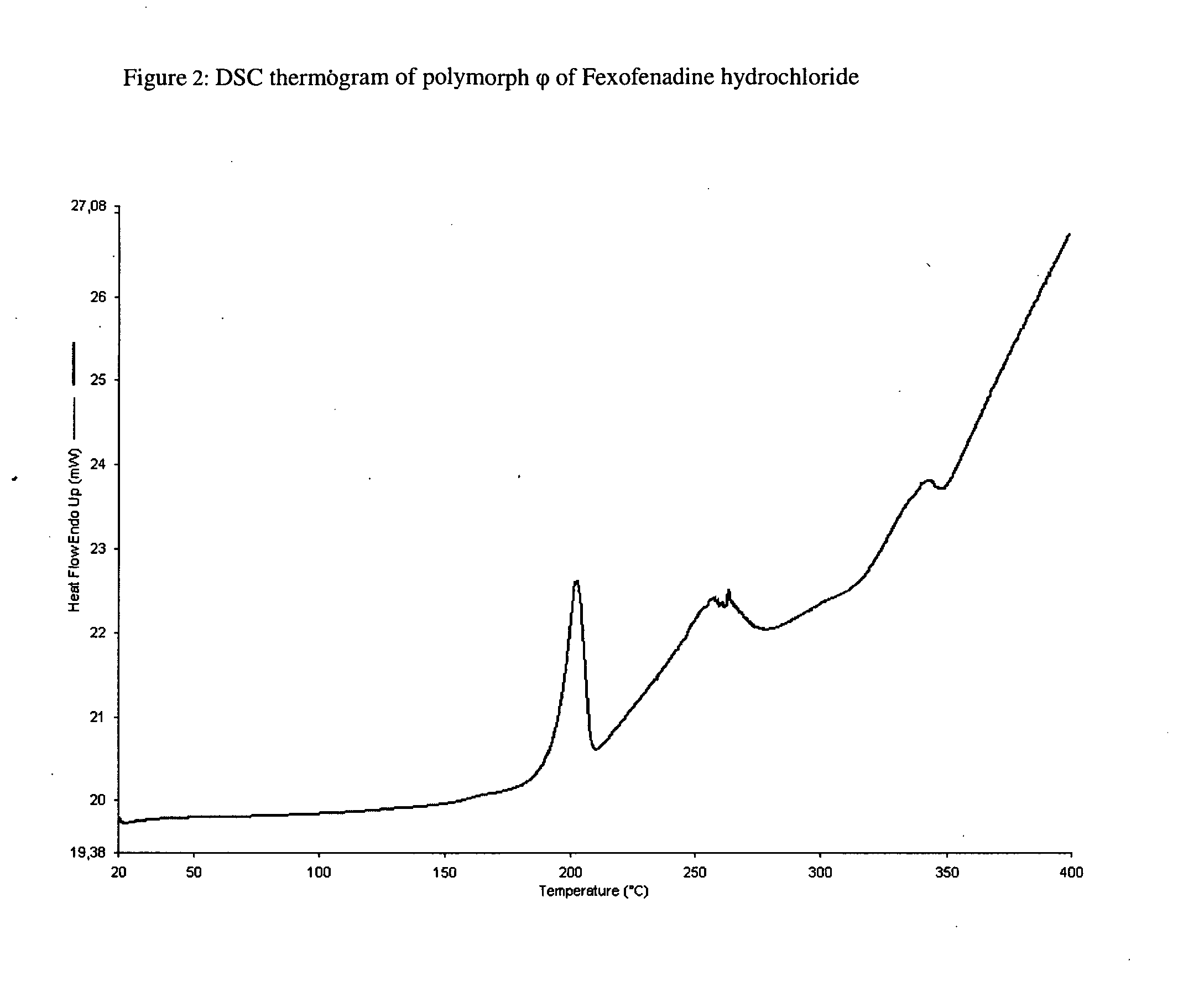
Polymorphic form of fexofenadine hydrochloride, intermediates and process for its preparation
InactiveUS20130059886A1Easy to handleComposition is stableBiocideOrganic chemistryAntihistamines drugsFexofenadine Hydrochloride
The present invention relates to a novel polymorphic form of Fexofenadine hydrochloride, to a process for preparing it, to pharmaceutical compositions containing it, as well as its use. The invention also relates to intermediates useful for the preparation of Fexofenadine hydrochloride, antihistamine drug used in the treatment of allergy symptoms.
Owner:CHEMELECTIVA
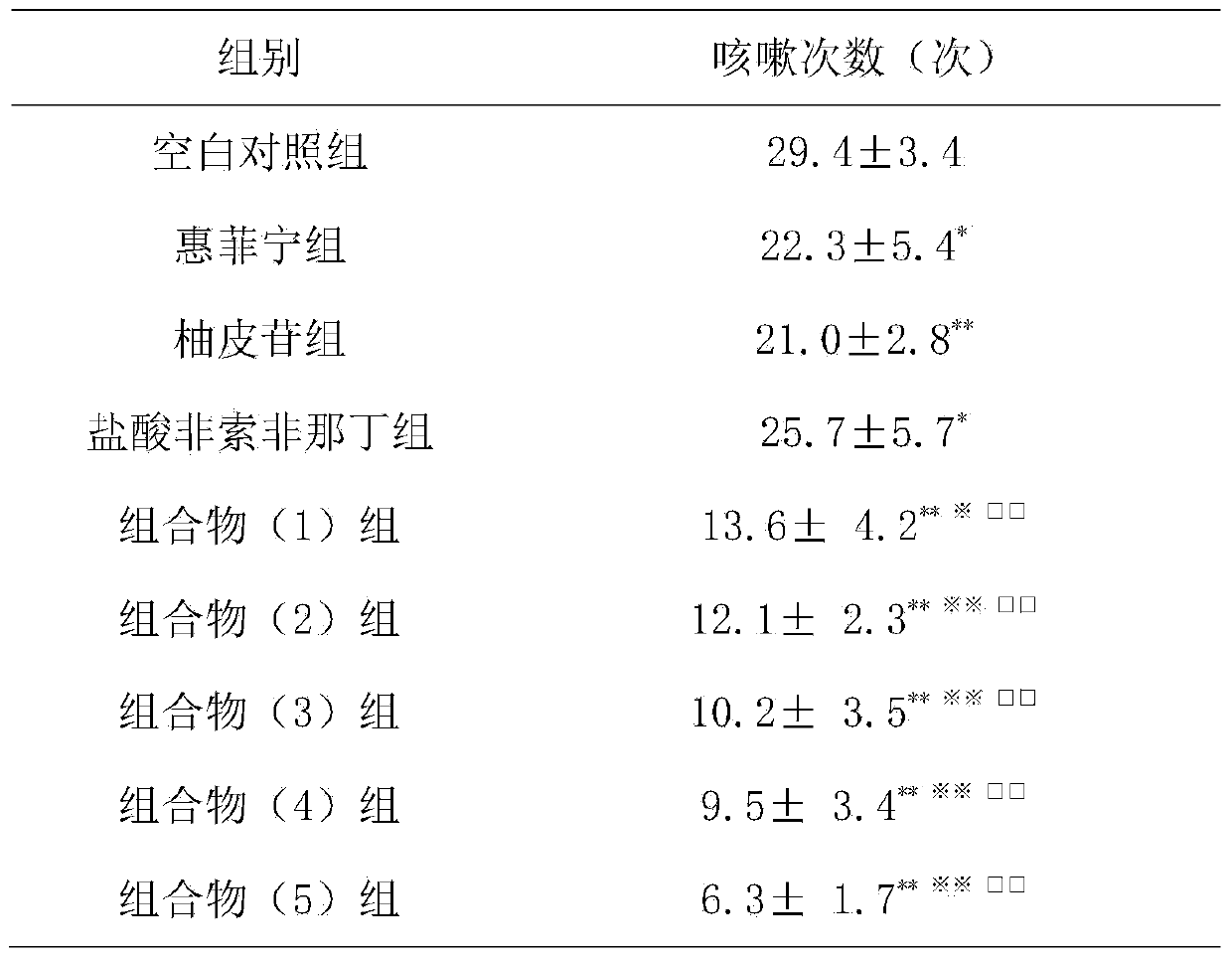
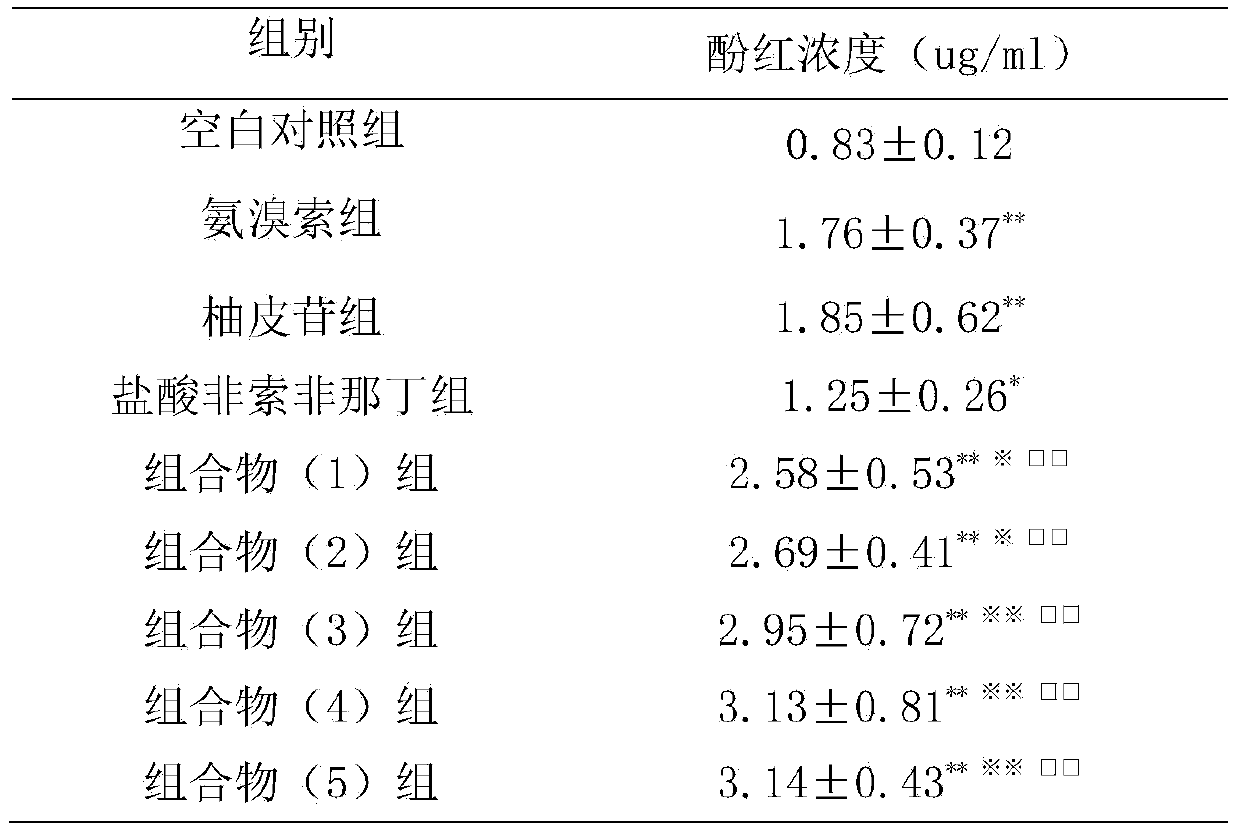
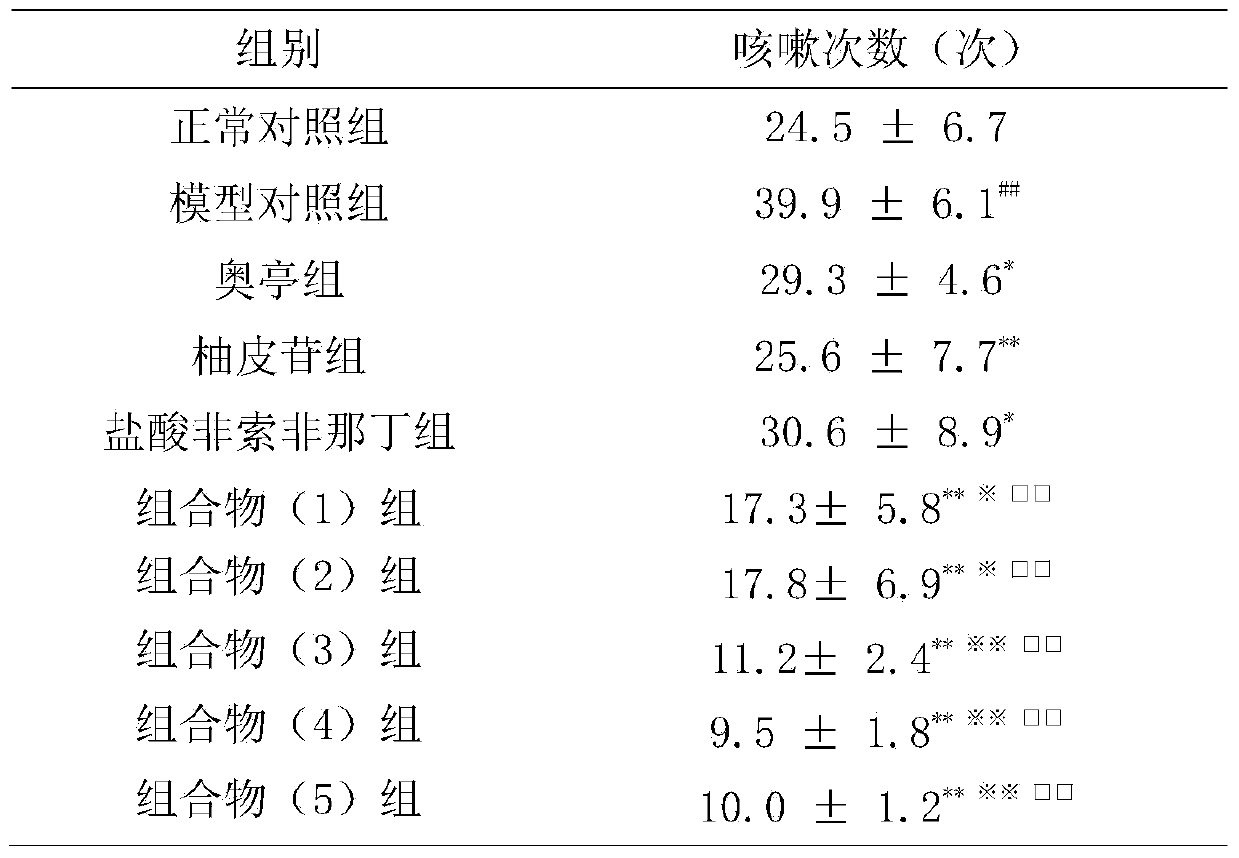
Naringin and fexofenadine hydrochloride drug composition and preparation thereof
InactiveCN104188998ACompound curative effect is excellentAntipyreticAnalgesicsNaringinCough variant asthma
The invention relates to a naringin drug composition for relieving cough, eliminating phlegm and relieving asthma and a preparation thereof. The naringin drug composition is characterized in that the naringin drug composition contains naringin and fexofenadine hydrochloride, the content per unit of the naringin in each compounding agent (preparation) is 27.5-275 mg, and the content per unit of the fexofenadine hydrochloride is 30-300 mg; in the composition, the preferred mass ratio of the naringin to the fexofenadine hydrochloride is 1 to 1, and the preferred dosage of the naringin and the fexofenadine hydrochloride in each compounding agent (preparation) are 40 mg and 40 mg respectively. The naringin drug composition and the preparation thereof have the benefits that with the adoption of the drug composition, the cough and the phlegm caused by various reasons as well as the cough variant asthma can be treated; the drug composition can be added with conventional excipients and then can be prepared into a drug for relieving cough, eliminating phlegm and relieving asthma by adopting any conventional methods; the drug effect generated by the composition is better than that generated when the naringin or the fexofenadine hydrochloride is independently used.
Owner:SUN YAT SEN UNIV
Synthetic process of fexofenadine hydrochloride
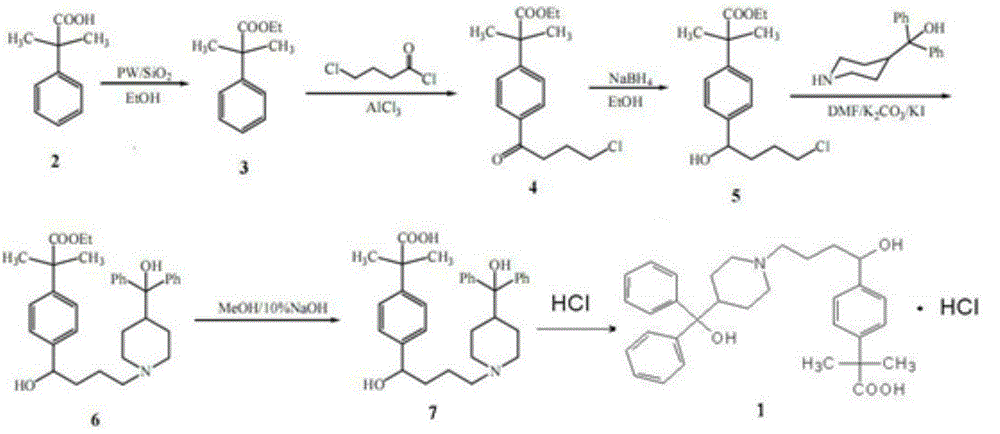
ActiveCN103333100AReduce manufacturing costFew synthetic stepsOrganic chemistryEthyl phenylacetateAzacyclonol
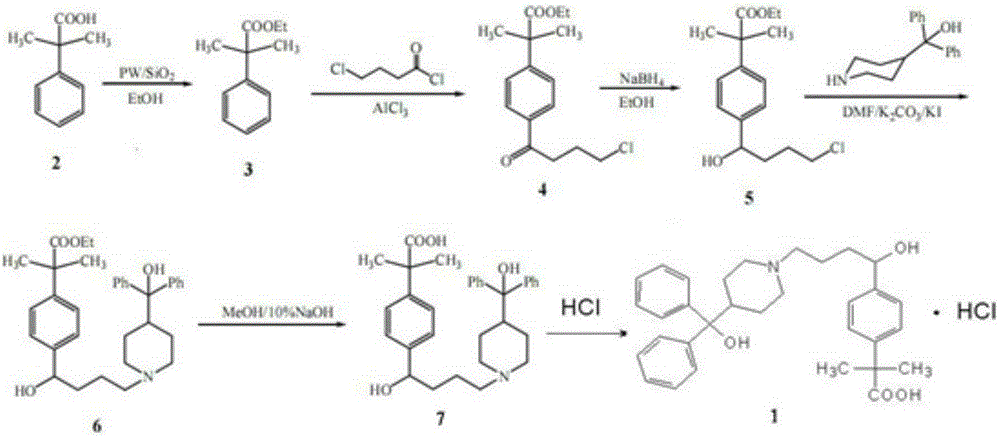
The invention discloses a synthetic process of fexofenadine hydrochloride. The synthetic process of fexofenadine hydrochloride comprises the following steps of: with alpha, alpha-dimethyl phenylacetic acid as a raw material, carrying out an esterification reaction on alpha, alpha-dimethyl phenylacetic acid and absolute ethyl alcohol under the catalysis of a silica gel loaded phosphotungstic acid (PW12 / SiO2) solid acid catalyst to obtain alpha, alpha-dimethyl ethyl phenylacetate; carrying out Friedel-Grafts reaction on alpha, alpha-dimethyl ethyl phenylacetate and 4-chlorobutyryl chloride to obtain alpha, alpha-dimethyl-4-(4-chloro-1-oxo butyl) ethyl phenylacetate; reducing by virtue of sodium borohydride in 95% ethyl alcohol to obtain alpha, alpha-dimethyl-4-(4-chloro-1-hydroxyl butyl) ethyl phenylacetate; and carrying out N-alkylation reaction on alpha, alpha-dimethyl-4-(4-chloro-1-hydroxyl butyl) ethyl phenylacetate and alpha, alpha-dimethyl-4-piperidine methyl alcohol in DMF (dimethyl formamide) for 24 hours at the temperature of 80 DEG C to obtain alpha, alpha-dimethyl-4-[1-hydroxyl-4-[4-(hydroxyl diphenylmethyl)-1-piperidyl]-butyl] ethyl phenylacetate, and then carrying out alkali hydrolysis and salification by virtue of hydrochloric acid, so that fexofenadine hydrochloride is obtained. The synthetic process of fexofenadine hydrochloride is high in yield and low in cost, produces less pollution and is applicable to industrial mass production.
Owner:CHIZHOU DONGSHENG PHARMA
Fexofenadine hydrochloride orally disintegrating tablet and preparation method thereof
ActiveCN101822646BSolve the problem of disintegrationSolves bitter tastePill deliveryPharmaceutical non-active ingredientsOrally disintegrating tabletDissolution
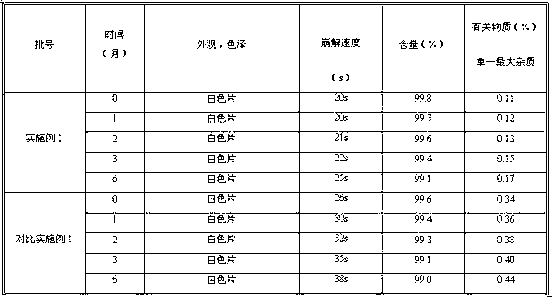
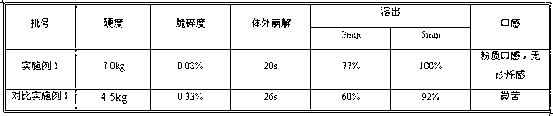
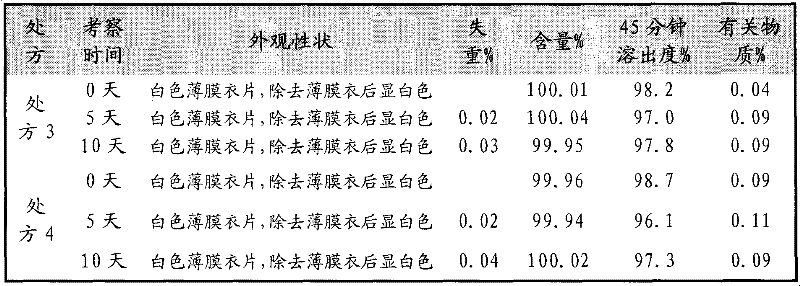
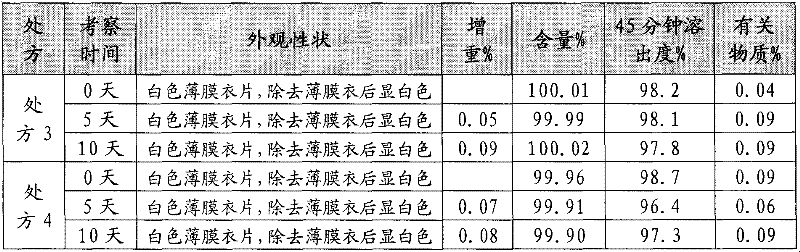
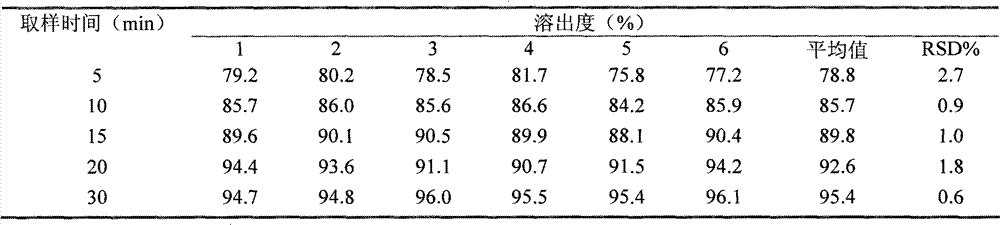
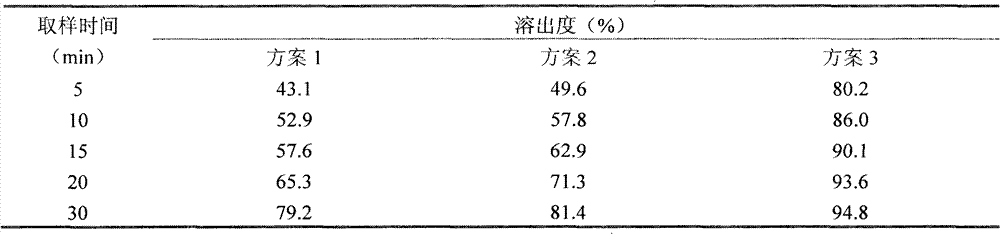
The invention discloses a fexofenadine hydrochloride orally disintegrating tablet, which consists of fexofenadine hydrochloride, a composite disintegrating agent, a filling agent, a lubricating agent and a composite flavoring agent. The fexofenadine hydrochloride orally disintegrating tablet is characterized in that the composite disintegrating agent is a highly efficient disintegrating agent, the highly efficient disintegrating agent is added internally and externally; and the composite flavoring agent is also added internally and externally. The orally disintegrating tablet of the inventionhas more excellent quality, can completely disintegrate in the oral cavity within 30 seconds, and has no gravel sense and no bitterness, and the dissolution can be more than 80 percent when determining the dissolution with water as the medium for ten minutes.
Owner:FUXIN LONGRUI CHEM CO LTD
Crystalline forms of fexofenadine and its hydrochloride
InactiveUS7700779B2Cost-effective and commercially viableBiocideOrganic chemistryFexofenadine HydrochlorideHydrochloride
The present invention is related to novel polymorph of Fexofenadine and Fexofenadine hydrochloride of formula 1 and process of preparation thereof. The present invention is also directed to provide pure novel polymorphs of Fexofenadine and its hydrochloride by a simple process which is cost effective, commercially viable and environment friendly.
Owner:DR REDDYS LAB LTD
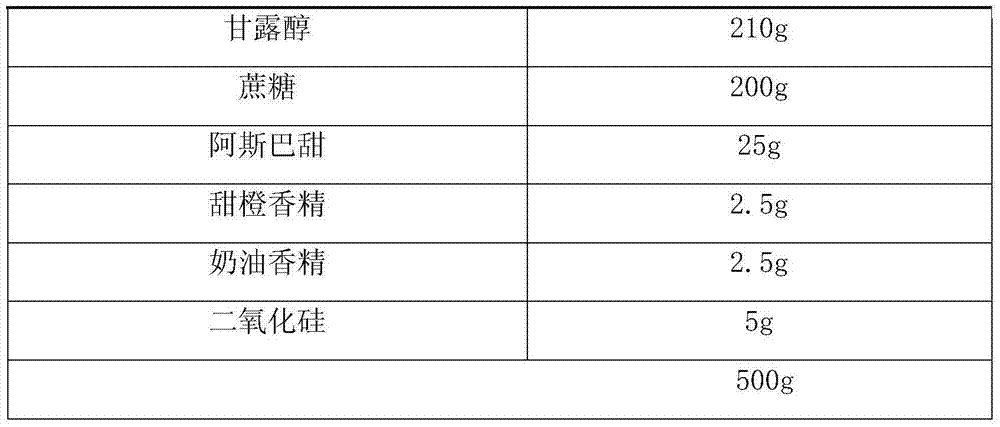
Fexofenadine hydrochloride dry suspension preparation and preparation method thereof
ActiveCN106806346AImprove stabilityImprove liquidityGranular deliveryImmunological disordersHigh absorptionPhosphoric acid
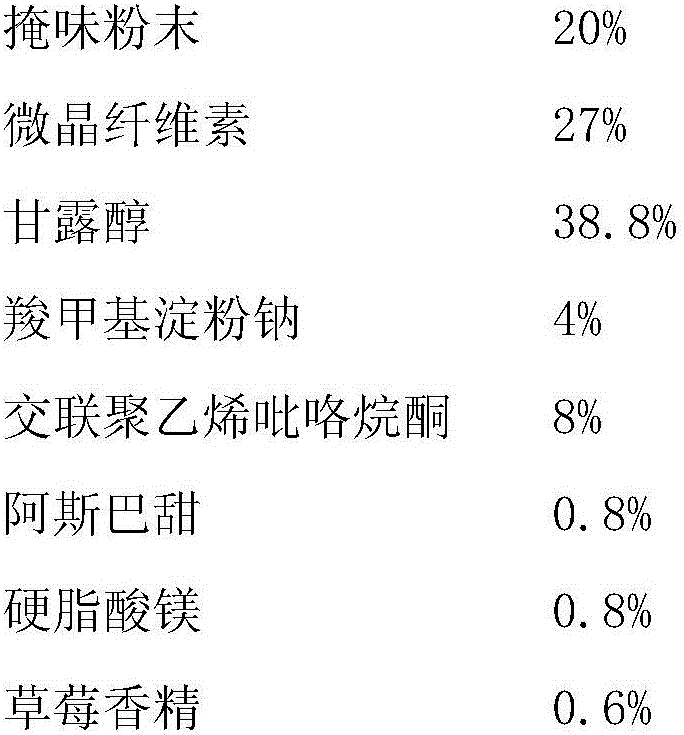
The invention discloses a fexofenadine hydrochloride dry suspension preparation and a preparation method thereof. The preparation comprises the following components in percentage by mass: 0.8-4% of fexofenadine hydrochloride, 80-93% of filler, 0.2-1.2% of sweetening agent, 0.5-1% of suspending agent, 0.2-2% of flavoring agent, 0.2-5% of taste masking agent, 0-0.6% of wetting agent and 1.5-10% of citric acid buffer salt or phosphoric acid buffer salt, totaling 100%. The fexofenadine hydrochloride dry suspension preparation is prepared by wet-process granulation. The fexofenadine hydrochloride dry suspension preparation has the advantages of high stability, favorable flowability and high storability. The suspension prepared from the preparation has the advantages of high absorption speed, high bioavailability, favorable mouthfeel and high stability, and is convenient to take.
Owner:SINOPHARM GUANGDONG GLOBAL PHARMA CO LTD +1
Preparation method for fexofenadine hydrochloride-containing medicinal composition
InactiveCN101884628ADefinite curative effectEfficient responseRespiratory disorderImmunological disordersCross-linkMedicine
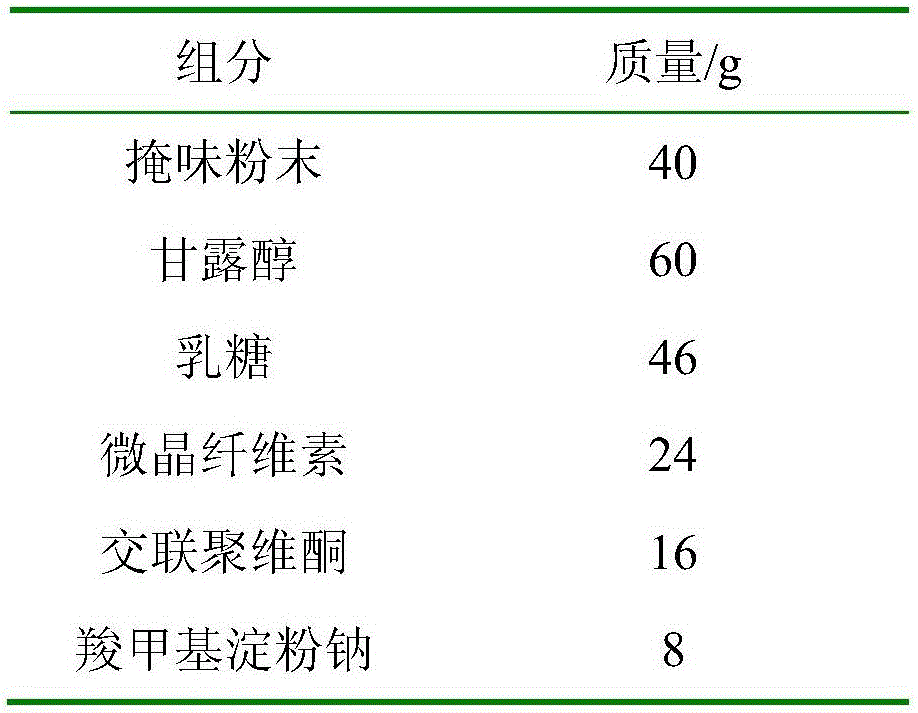
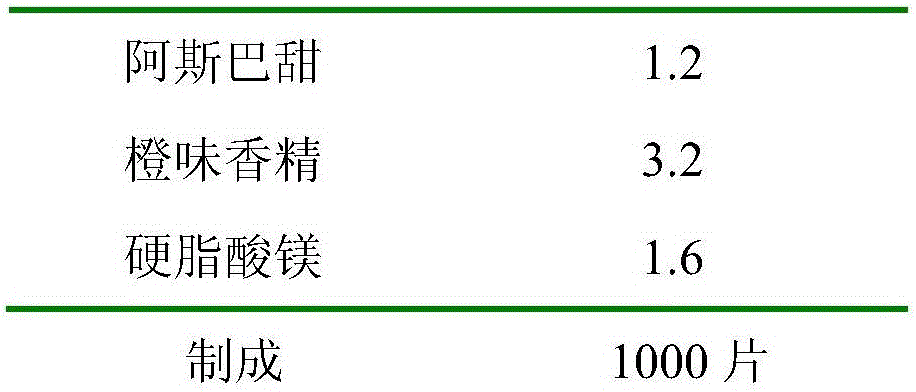
The invention relates to a preparation method for a fexofenadine hydrochloride-containing medicinal composition. The method is characterized by comprising the following steps of: (1), respectively sieving a main medicament, namely fexofenadine hydrochloride, and auxiliary materials, namely lactose, starch, hydroxypropylcellulose, cross-linked povidone and magnesium stearate for later use; (2), preparing 5 percent (g / l) solution by dissolving polyvinylpyrrolidone (k30) into ethanol solution; (3), uniformly mixing fexofenadine hydrochloride, and the auxiliary materials, namely lactose, starch and hydroxypropylcellulose; (4), uniformly mixing appropriate amount of (2) and (3), and making the mixture into granules with a 14-mesh nylon sieve, drying the granules at 60 to 65 DEG C; (5), mixing the cross-linked povidone and the magnesium stearate with (4), modifying the grains by using a 14-mesh stainless steel net, and uniformly mixing; and (6), detecting an intermediate, and tabletting and coating. The use amount of an inactive component is small; the mixing difficulty of fexofenadine hydrochloride is low so that the energy consumption and production cost are lowered and the utilization rate of equipment is comparatively high on the one hand; and tablets are small enough to be carried and used conveniently by patients on the other hand.
Owner:ZHEJIANG WAN SHENG PHARMA CO LTD
Fexofenadine Polymorphs and Processes of Preparing the Same
InactiveUS20090221830A1Easy to prepareKeep dryBiocideSenses disorderMedicinal chemistryFexofenadine hcl
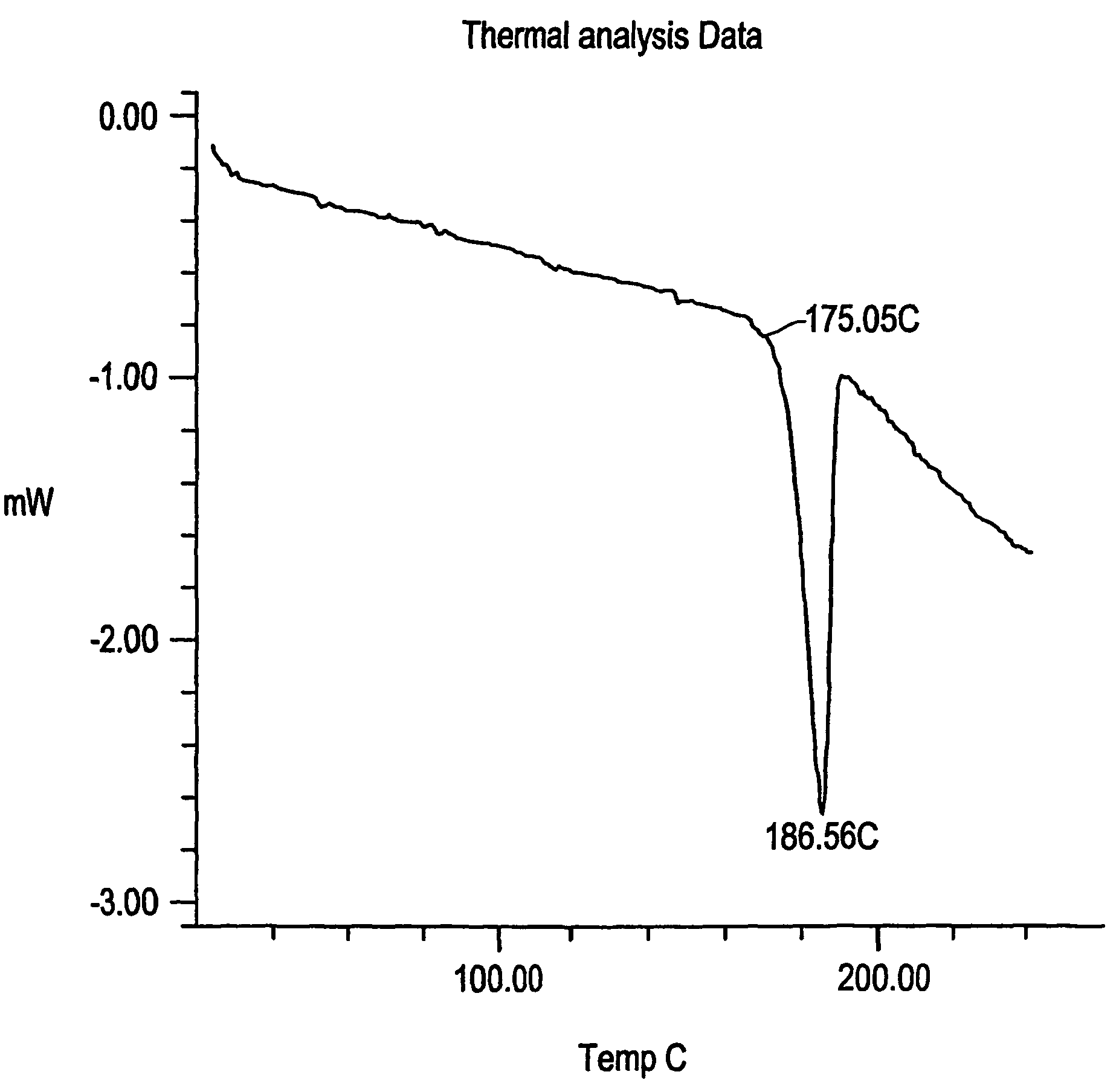
Anhydrous crystalline fexofenadine hydrochloride Form C, crystalline fexofenadine acetate monohydrate Form D, crystalline fexofenadine acetate dihydrate Form E and crystalline fexofenadine free base monohydrate Form F, processes of preparing the same, pharmaceutical compositions thereof, therapeutic uses thereof and methods of treatment therewith.
Owner:CIPLA LTD
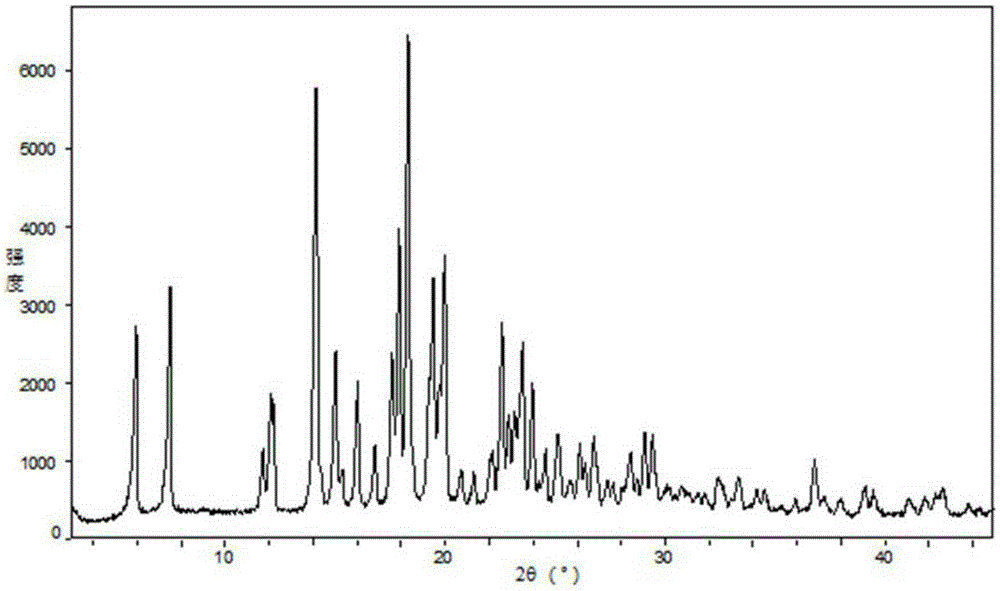
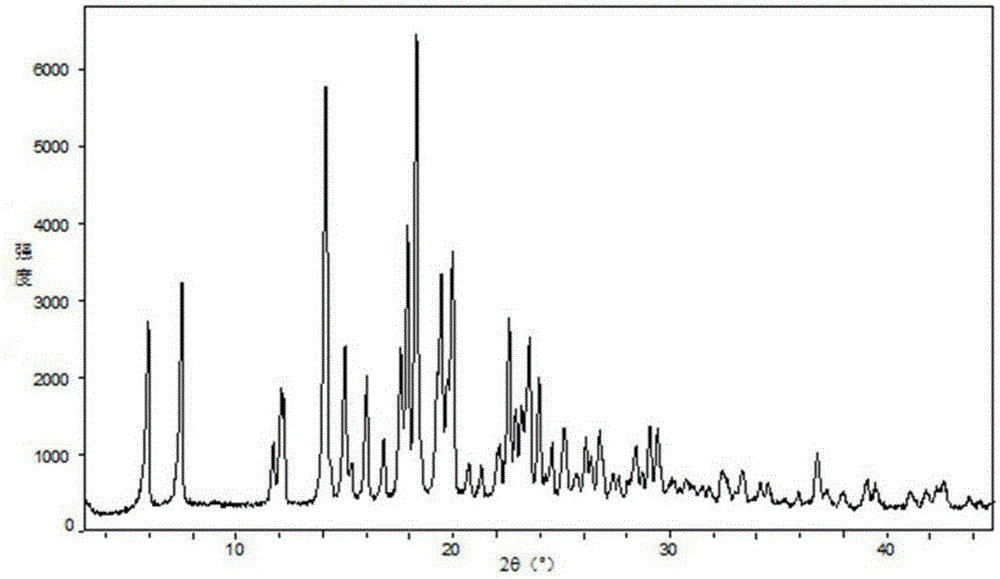
Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride
The present invention is related to novel polymorph of Fexofenadine and Fexofenadine hydrochloride of formula 1 and process of preparation thereof. The present invention is also directed to provide pure novel polymorphs of Fexofenadine and its hydrochloride by a simple process which is cost effective, commercially viable and environment friendly.
Owner:DR REDDYS LAB LTD
Anthistamine-decongestant pharmaceutical compositions
InactiveUS8263124B2BiocidePharmaceutical non-active ingredientsAntihistamines decongestantsDecongestant
The present invention relates to pharmaceutical compositions of antihistamine-decongestant combination. Specifically the invention relates to bilayered tablet formulation comprising antihistaminic decongestant combination. More specifically present invention relates to the novel polymorph of fexofenadine or pharmaceutically accepted salts thereof, with at least one decongestant in the form of bilayered tablet. The preferred polymorphs are polymorph A and polymorph X of fexofenadine hydrochloride.
Owner:DR REDDYS LAB LTD
Application of fexofenadine hydrochloride in preparation of medicine for treating schizophrenia
ActiveCN105456265AChange negative symptomsImprove cognitive abilityNervous disorderHeterocyclic compound active ingredientsNegative symptomCurative effect
The invention discloses application of fexofenadine hydrochloride in preparation of a medicine for treating schizophrenia. The medicine further can be prepared from risperidone. The medicine is prepared from, by mass, 10-180 parts of the fexofenadine hydrochloride and 0.5-3 parts of the risperidone. The medicine can obviously change the negative symptoms of the schizophrenia and achieve the obvious treatment effect compared with a medicine prepared from the risperidone alone; the cognitive ability of a schizophrenia patient is improved to 30%-40% by using the existing medicine prepared from the risperidone alone; according to the medicine, the fexofenadine hydrochloride and the risperidone are combined together, the synergistic effect is achieved, and the cognitive ability of the schizophrenia patient can be improved to 90%.
Owner:韩自勤
A kind of fexofenadine hydrochloride tablet and preparation method thereof
ActiveCN103393613BReduce dosageReduce manufacturing costPill deliveryPharmaceutical non-active ingredientsCitric acidFexofenadine Hydrochloride
Owner:南京易亨制药有限公司
Fexofenadine hydrochloride tablet and preparation method thereof
ActiveCN103393613AReduce dosageReduce manufacturing costPharmaceutical non-active ingredientsPill deliveryCitric acidFexofenadine Hydrochloride
The invention discloses a fexofenadine hydrochloride tablet and a preparation method thereof. The preparation is prepared from solid dispersion and pharmaceutical acceptable auxiliary materials by the procedures of evenly mixing and directly tabletting, wherein the solid dispersion is obtained by dissolving fexofenadine hydrochloride and citric acid into ethanol, drying and removing ethanol. According to the tablet disclosed by the invention, the dosage of a disintegrating agent is reduced under the premise of ensuring that the drug is rapidly dissolved out; the production cost is reduced; meanwhile, the fexofenadine hydrochloride tablet is simple in preparation technology, and good in surface after accelerated inspection.
Owner:南京易亨制药有限公司
Synthetic method of a fexofenadine hydrochloride
InactiveCN101585804AHigh yieldReduce pollutionOrganic chemistryImmunological disordersMethacrylate2-methylpropanoic acid
The invention discloses a synthetic method of a fexofenadine hydrochloride, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; refluxing the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an alkaline alcohol solvent and adjusting pH to obtain 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid; reacting the 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid in HCl for 20-30h at 60-100 DEG C and recrystallizing to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid in the hydrochloric acid solution of absolute alcohol to prepare the 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, and finally obtaining the target product of the invention through routine techniques. The invention has the advantages of high yield, freeness of meta-isomers and amide impurities, little pollution and applicable industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
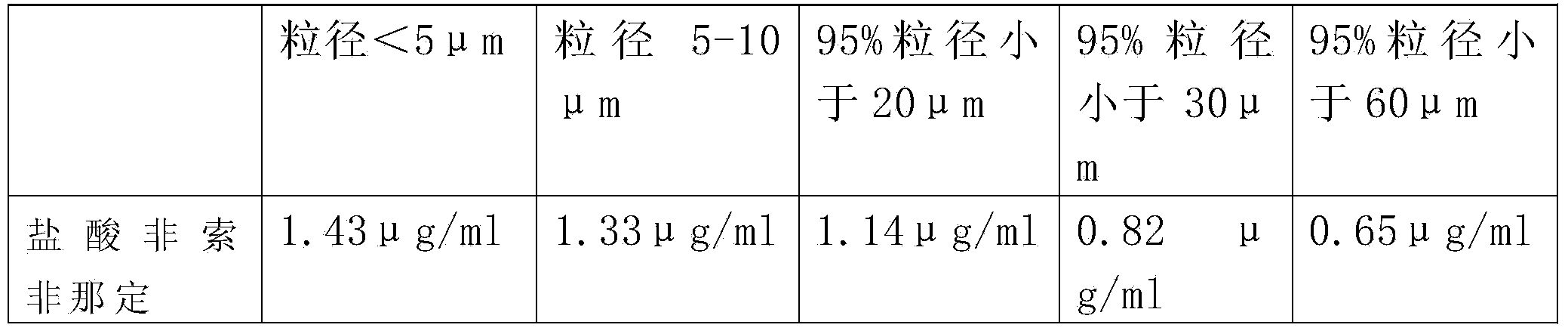
Pharmaceutical composition containing micronized fexofenadine hydrochloride
ActiveCN104257611AReduce in quantityExcellent in vitro dissolutionPowder deliveryPill deliveryCurative effectCrowds
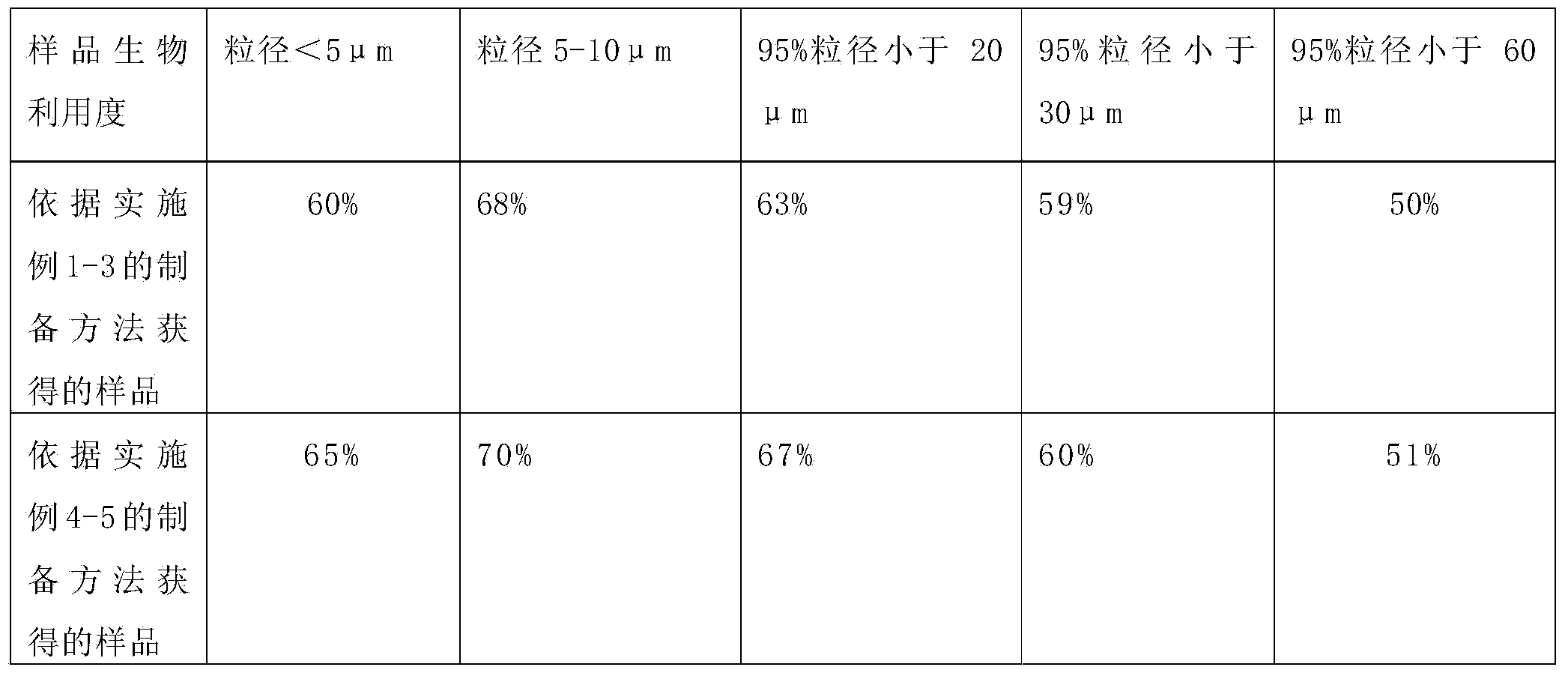
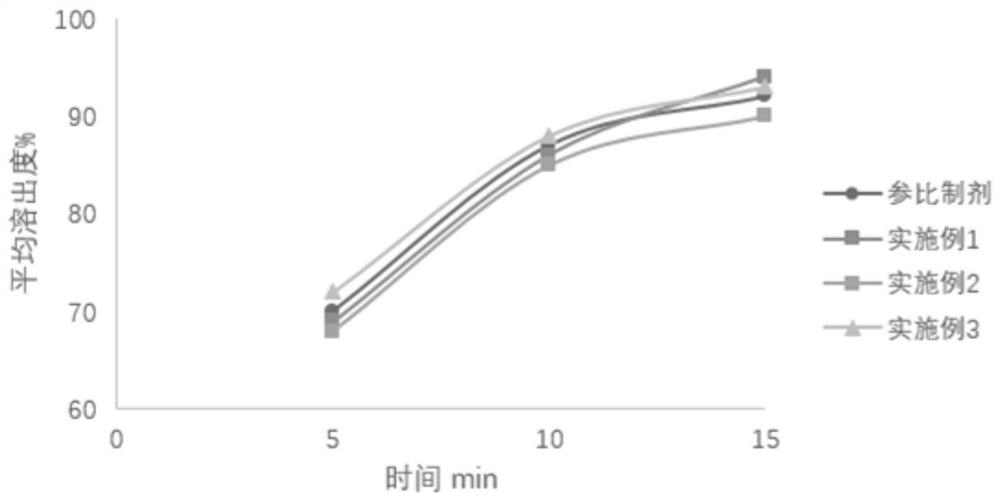
The invention provides micronized fexofenadine hydrochloride. The particle size of fexofenadine hydrochloride at 95% cumulative volume is 60mu m or below. The invention also provides a method for preparing the micronized fexofenadine hydrochloride, a pharmaceutical composition containing the micronized fexofenadine hydrochloride, as well as a preparation method of the pharmaceutical composition. According to the invention, a solid preparation by taking the micronized fexofenadine hydrochloride as the active ingredient has excellent dissolution rate in vitro and has high dissolution characteristics under various pH conditions, the corresponding effects of the medicine in vivo in different crowds can be effectively guaranteed, and the problem that the bioavailability of the medicine in vivo is reduced so as to influence the curative effect because the conventional fexofenadine hydrochloride medicine is difficult to dissolve in an in-vivo gastrointestinal environment is solved.
Owner:KUSN ROTAM REDDY PHARMA
Taste masking preparation and preparing method thereof
InactiveCN105997910ASmall granularityReduce usagePharmaceutical non-active ingredientsPill deliverySolubilityCellulose acetate phthalate
The invention discloses a taste masking preparation and a preparing method thereof, belongs to the field of pharmaceutic preparations, and aims at providing a coating-free taste masking preparation of fexofenadine hydrochloride and a preparing method thereof. The preparing method of the taste masking preparation includes the steps that fexofenadine hydrochloride is dispersed into a solution of a taste masking material, and the taste masking material is separated out and precipitated on the surfaces of medicine particles through stirring and mixing or by adding a neutral or alkaline aqueous solution for dilution. The taste masking material is an enteric solubility material or stomach-solubility-type polyacrylic resin. The enteric solubility material comprises but is not limited to one or more of methacrylic acid copolymers, cellulose acetate phthalate, hydroxypropyl methylcellulose phthalate and polyvinyl acetate phthalate. By means of the taste masking preparation and the preparing method, an original coating taste masking method is avoided, coating equipment is avoided, the production technology process is simple and convenient, cost is low, and the safety of the product is high; the product has the advantages of being small in tablet weight, free of coating and masticable.
Owner:BEIJING UNIV OF CHEM TECH
Fexofenadine hydrochloride oral disintegrating drug composition
InactiveCN102512389BHigh yieldReduce market riskPharmaceutical non-active ingredientsPill deliveryBioavailabilityPharmaceutical Substances
Owner:JIANGSU GAOSHIDA ELECTRIC TOOL CO LTD
A kind of synthesis technique of fexofenadine hydrochloride
ActiveCN103333100BReduce manufacturing costFew synthetic stepsOrganic chemistryEthyl phenylacetateAzacyclonol
The invention discloses a synthetic process of fexofenadine hydrochloride. The synthetic process of fexofenadine hydrochloride comprises the following steps of: with alpha, alpha-dimethyl phenylacetic acid as a raw material, carrying out an esterification reaction on alpha, alpha-dimethyl phenylacetic acid and absolute ethyl alcohol under the catalysis of a silica gel loaded phosphotungstic acid (PW12 / SiO2) solid acid catalyst to obtain alpha, alpha-dimethyl ethyl phenylacetate; carrying out Friedel-Grafts reaction on alpha, alpha-dimethyl ethyl phenylacetate and 4-chlorobutyryl chloride to obtain alpha, alpha-dimethyl-4-(4-chloro-1-oxo butyl) ethyl phenylacetate; reducing by virtue of sodium borohydride in 95% ethyl alcohol to obtain alpha, alpha-dimethyl-4-(4-chloro-1-hydroxyl butyl) ethyl phenylacetate; and carrying out N-alkylation reaction on alpha, alpha-dimethyl-4-(4-chloro-1-hydroxyl butyl) ethyl phenylacetate and alpha, alpha-dimethyl-4-piperidine methyl alcohol in DMF (dimethyl formamide) for 24 hours at the temperature of 80 DEG C to obtain alpha, alpha-dimethyl-4-[1-hydroxyl-4-[4-(hydroxyl diphenylmethyl)-1-piperidyl]-butyl] ethyl phenylacetate, and then carrying out alkali hydrolysis and salification by virtue of hydrochloric acid, so that fexofenadine hydrochloride is obtained. The synthetic process of fexofenadine hydrochloride is high in yield and low in cost, produces less pollution and is applicable to industrial mass production.
Owner:CHIZHOU DONGSHENG PHARMA
Composition of fexofenadine hydrochloride and microcrystalline cellulose and preparation method thereof
ActiveCN101843616BSignificant clinical effectDisintegrates quicklyPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMagnesium stearateCompressibility
Owner:西安万隆制药股份有限公司
Fexofenadine hydrochloride dry suspension and preparation method thereof
PendingCN114681616AGreat tasteGood suspensionPharmaceutical non-active ingredientsRespiratory disorderPharmaceutical drugDrug activity
The invention discloses a fexofenadine hydrochloride dry suspension and a preparation method thereof. The fexofenadine hydrochloride dry suspension takes fexofenadine hydrochloride as an active pharmaceutical ingredient, and comprises the following components in percentage by mass: 2 to 8 percent of fexofenadine hydrochloride, 89 to 95 percent of filler, 0.5 to 2.0 percent of suspending aid, 0.02 to 0.10 percent of wetting agent and 0.20 to 1.00 percent of flavoring agent. The fexofenadine hydrochloride dry suspension is prepared in a wet granulation mode, the prepared fexofenadine hydrochloride dry suspension is good in taste, good in patient compliance and high in stability, and the drug dissolution rate can be further improved.
Owner:SHANGHAI INST OF PHARMA IND +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride](https://images-eureka.patsnap.com/patent_img/d0b45a86-06d0-4b68-ba02-3e752722a29b/US20040077683A1-20040422-C00001.png)
![Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride](https://images-eureka.patsnap.com/patent_img/d0b45a86-06d0-4b68-ba02-3e752722a29b/US20040077683A1-20040422-D00001.png)
![Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-1-hydroxybutyl]-$g(a)-dimethylbenzene acetic acid and its hydrochloride](https://images-eureka.patsnap.com/patent_img/d0b45a86-06d0-4b68-ba02-3e752722a29b/US20040077683A1-20040422-D00002.png)



![Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride](https://images-eureka.patsnap.com/patent_img/e62540c9-e4ed-4635-8aeb-0b98a2808d74/c0182337900171.PNG)
![Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride](https://images-eureka.patsnap.com/patent_img/e62540c9-e4ed-4635-8aeb-0b98a2808d74/c0182337900181.PNG)
![Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride Novel crystalline forms of 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-a, a-dimethylbenzene acetic acid and its hydrochloride](https://images-eureka.patsnap.com/patent_img/e62540c9-e4ed-4635-8aeb-0b98a2808d74/c0182337900191.PNG)