Method for preparing fexofenadine hydrochloride orally disintegrating tablet
A technology for fexofenadine hydrochloride and oral disintegrating tablets, which is applied in directions such as pharmaceutical formulations, medical preparations of inactive ingredients, and pill delivery, can solve the problems of increasing the difficulty of taking children's patients, and achieves high dissolution rate, The effect of fast disintegration time and long storage time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the process of preparing 1000 pieces of fexofenadine hydrochloride of 30mg / tablet
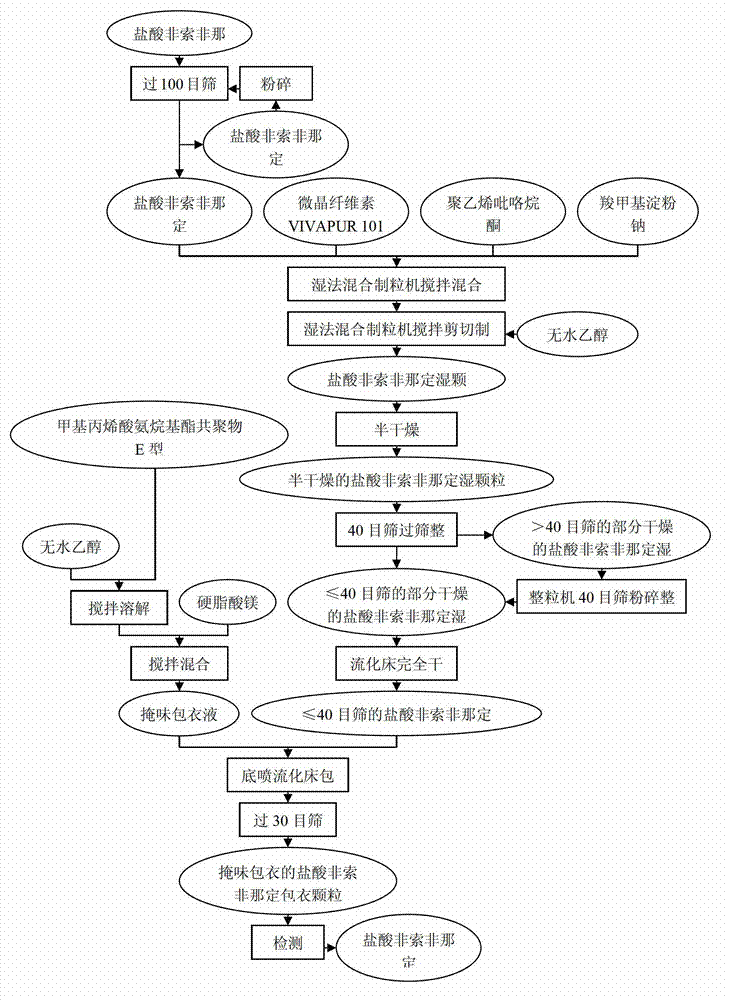
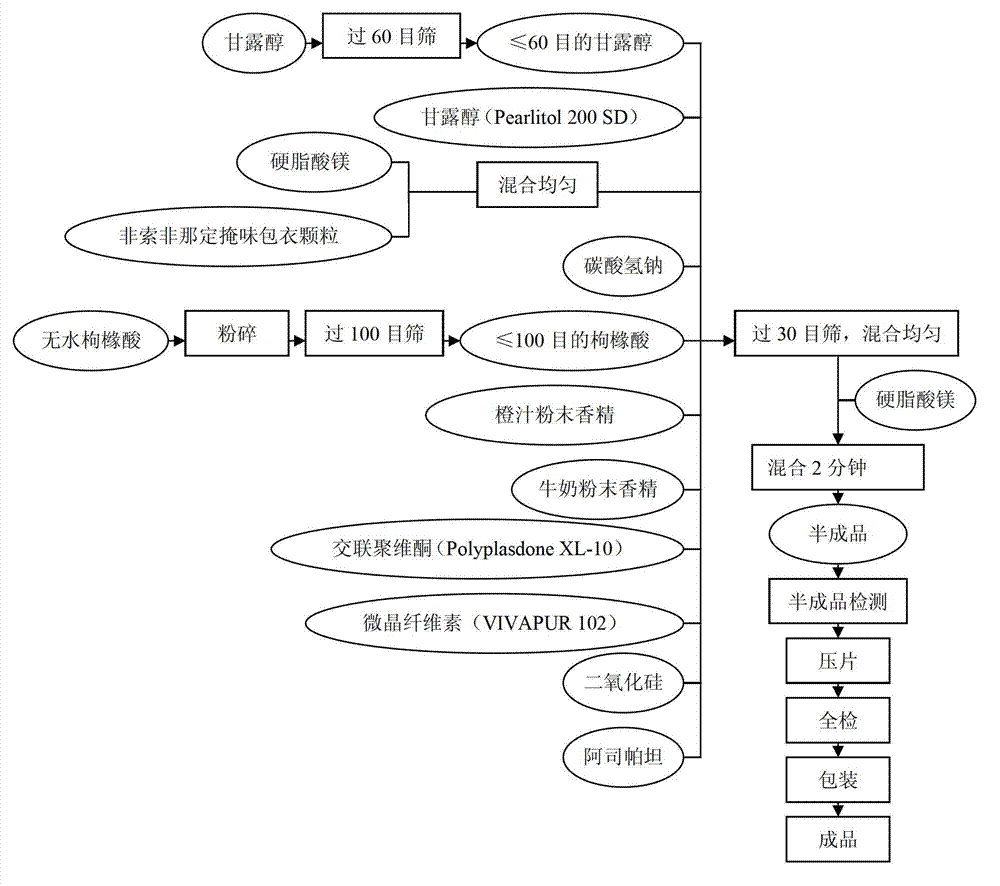
[0017] 1.1. Preparation process of granules for coating ( figure 1 )
[0018] 1.1.1. According to the prescription listed in Table 1 below, weigh various raw and auxiliary materials (except absolute ethanol) required for the preparation of fexofenadine hydrochloride wet granules, pass through a 100-mesh sieve, and mix evenly;
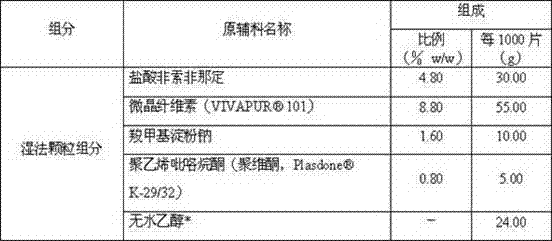
[0019] Table 1: Feed amount of each batch of wet granules
[0020]
[0021] *Removed during process
[0022] 1.1.2. Materials to be mixed in step 1.1.1: fexofenadine hydrochloride, microcrystalline cellulose (VIVAPUR® 101), sodium carboxymethyl starch and polyvinylpyrrolidone (povidone, Plasdone® K-29 / 32) Add it to the whole grain wet mixing granulator (purchased from Zhejiang Jianan Technology Co., Ltd., model LHS300), start shearing and mixing, the rotation speed of the stirring blade is 504rpm, the rotation speed of the granulation knife...
Embodiment 2
[0055] Example 2 At the same time, according to the Chinese patent application publication, the method disclosed in the publication number 10175679 is used to prepare Fexofer hydrochloride orally disintegrating tablets.
Embodiment 3
[0056] Example 3 At the same time, according to the Chinese patent application publication, the method disclosed in the publication number 101824646 was used to prepare Fexofer hydrochloride orally disintegrating tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com