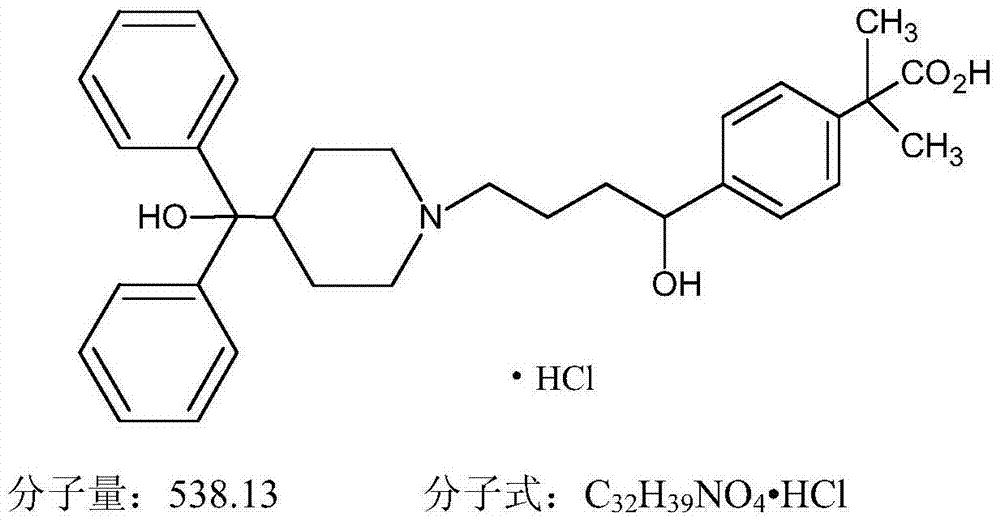
Pharmaceutical composition with fexofenadine hydrochloride and preparation method of pharmaceutical composition
A technology of fexofenadine and its composition, which is applied in the field of medicine and can solve problems such as difficult control of disintegration time limit and dissolution rate, inconvenient production, storage and transportation, and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
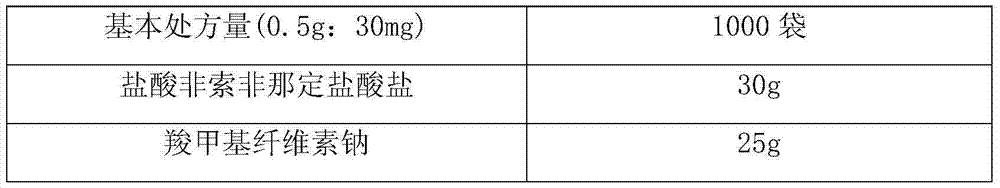
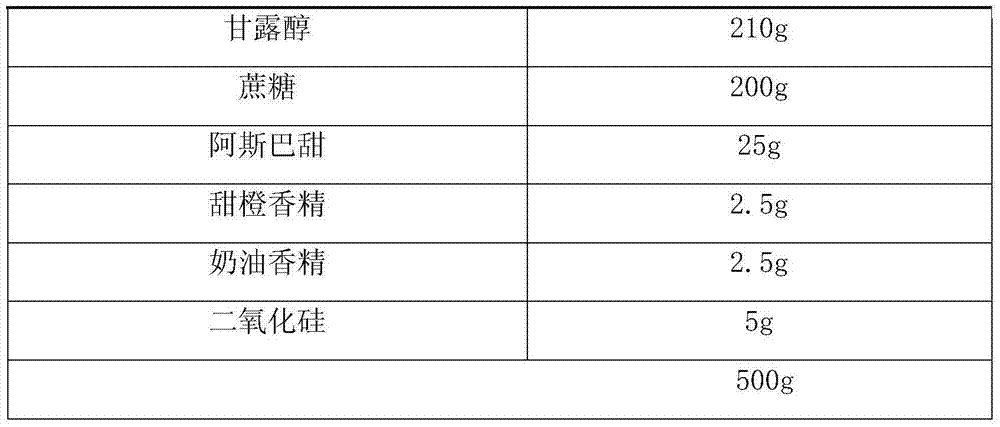
[0026] Product prescription (Table 1):
[0027]
[0028]
[0029] A total of 1000 bags of production process: pass fexofenadine hydrochloride and suspending agent through an 80-mesh sieve, pass each component of filler, sweetener, flavor and glidant through a 100-mesh sieve, and pass the sieved ingredients Mix evenly and dispense.
Embodiment 2
[0031] Product prescription (Table 2):
[0032]
[0033] A total of 1000 bags of production process: pass fexofenadine hydrochloride and suspending agent through an 80-mesh sieve, pass each component of filler, sweetener, flavor and glidant through a 100-mesh sieve, and pass the sieved ingredients Mix evenly and dispense.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com