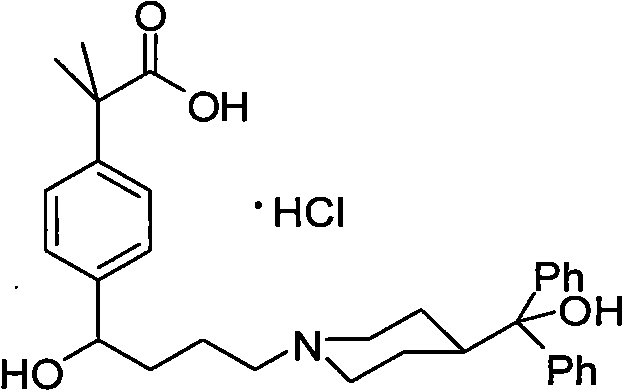
Synthetic method of fexofenadine hydrochloride
A technology of fexofenadine hydrochloride and its synthesis method, which is applied in the field of synthesis of fexofenadine hydrochloride, can solve the problems of difficult amide separation, reduced purity and yield, and low purity of fexofenadine hydrochloride, and achieves The effect of simple process route, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
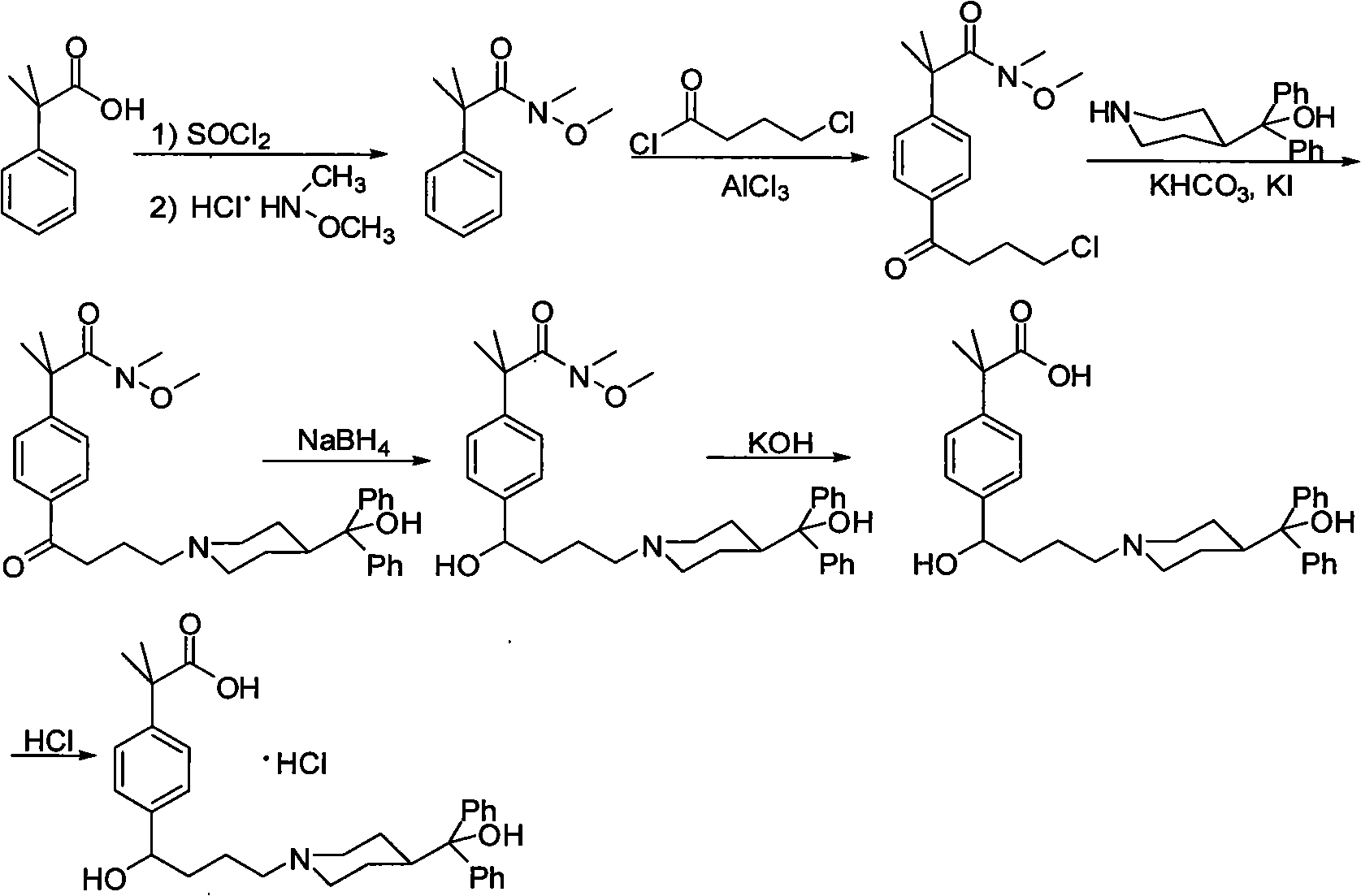
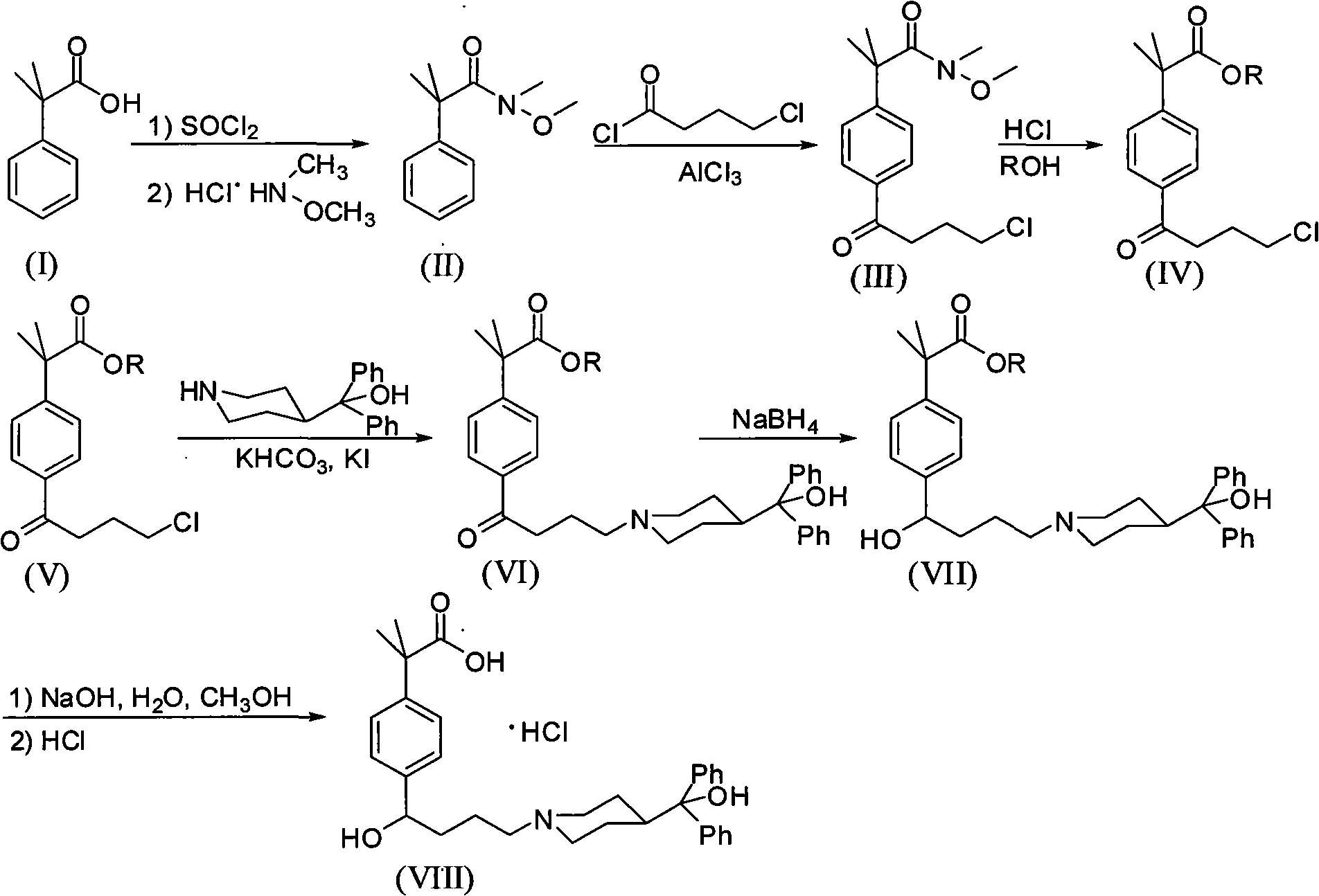
[0030]The present invention starts with N-methyl-N-methoxy-2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanamide (III) as the starting material. The synthetic method of N-methyl-N-methoxy-2-[4-(4-chlorobutyryl) phenyl]-2-methylpropionamide is prepared by prior art: 98.4 grams (0.6mol) α , α-dimethylphenylacetic acid was dissolved in 400 milliliters of toluene, 131 milliliters of thionyl chloride was added dropwise at 0°C, stirred at room temperature for 15 hours and then refluxed for 2 hours, and 100 milliliters of toluene and toluene were removed by vacuum distillation after the reaction was completed. Excess thionyl chloride, add 184.6 grams (1.34mol) of potassium carbonate, 58.5 grams (0.6mol) of N, O-dimethylhydroxylamine hydrochloride and 300 milliliters of water, stir and react at room temperature for 4 hours, after the completion of the reaction, the reaction 200 ml of 2N hydrochloric acid was added dropwise to the mixture, and the liquid was separated. The organic phase wa...
Embodiment 2
[0038] Using the N-methyl-N-methoxy-2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanamide prepared by the method of the above-mentioned Example 1 as the starting material, in 250 ml three Add 140 ml of anhydrous methanol to the flask, bubble dry hydrogen chloride gas at 0°C until saturated, and then add N-methyl-N-methoxy-2-[4-(4-chlorobutyryl)benzene Base]-2-methyl propionamide 20 grams (0.064mol), reflux reaction 24 hours after adding. After the reaction was completed, anhydrous methanol and hydrogen chloride were distilled off under reduced pressure, 200 ml of water and 200 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. The extracts containing the dichloromethane layer were combined, dried with anhydrous sodium sulfate, filtered, and the dichloromethane was distilled off under reduced pressure to obtain the product 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropane 16...
Embodiment 3
[0041] Using the N-methyl-N-methoxy-2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanamide prepared by the method of the above-mentioned Example 1 as the starting material, in 250 ml three Add 140 ml of anhydrous n-propanol to the flask, bubble dry hydrogen chloride gas at 0°C until saturated, then add N-methyl-N-methoxy-2-[4-(4-chlorobutyryl ) phenyl]-2-methyl propionamide 17.47 grams (0.056 mol), reflux reaction for 30 hours after adding. After the reaction was completed, anhydrous n-propanol and hydrogen chloride were evaporated under reduced pressure, 200 ml of water and 200 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. The extracts containing the dichloromethane layer were combined, dried with anhydrous sodium sulfate, filtered, and the dichloromethane was distilled off under reduced pressure to obtain the product 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropane 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com