Fexofenadine hydrochloride dry suspension and preparation method thereof
A technology of fexofenadine hydrochloride and dry suspension, which is applied in the field of fexofenadine hydrochloride dry suspension and its preparation, can solve problems such as excessive flavoring agent content, and achieve good suspending property, Good for storage and good taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
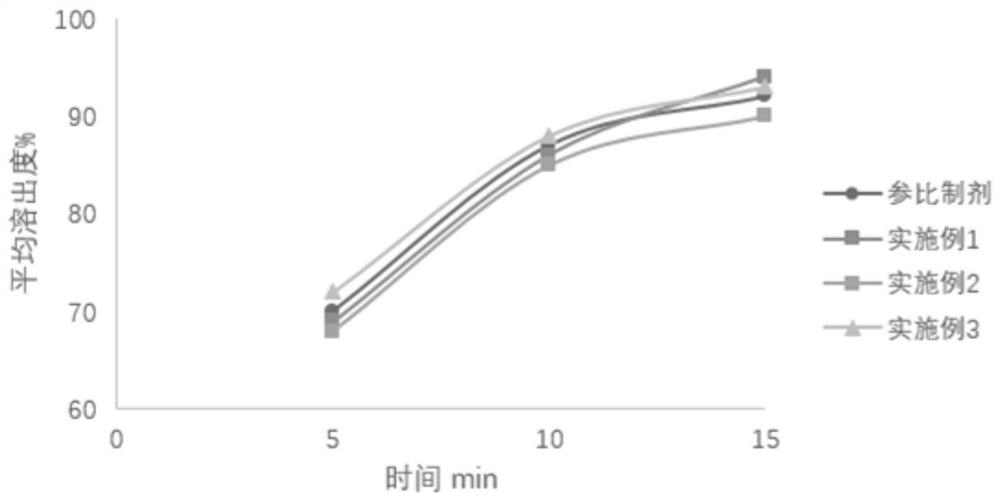
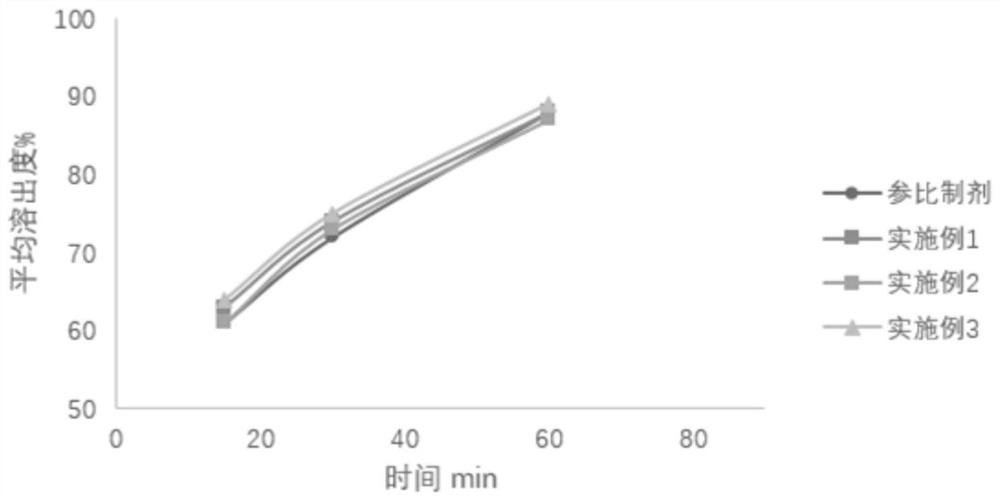
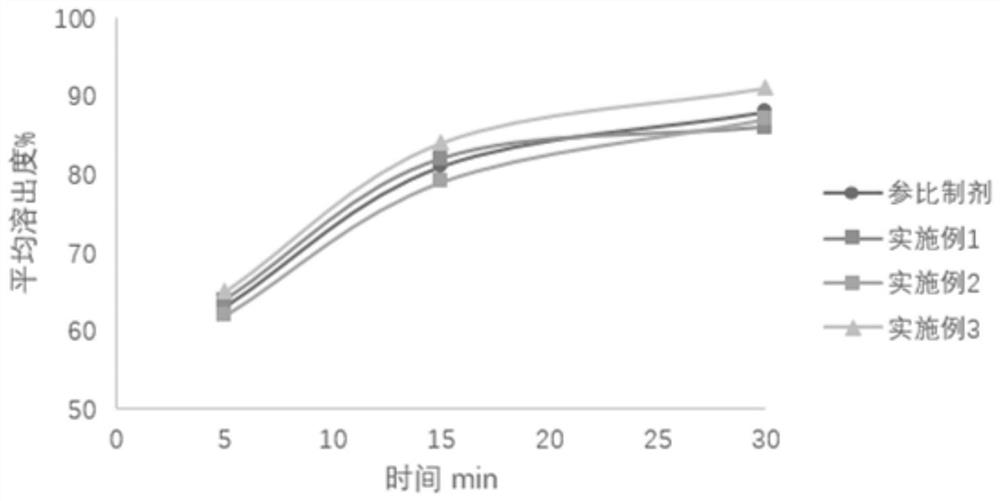
Image
Examples
Embodiment 1
[0042] The prescription of fexofenadine hydrochloride dry suspension is as follows in Table 1:
[0043] Table 1 embodiment 1 fexofenadine hydrochloride dry suspension prescription
[0044]
[0045]
[0046] Prepared by the following preparation method:
[0047] (1) Weigh sodium dioctyl sulfosuccinate and ethyl cellulose according to the recipe quantity, and use absolute ethanol to prepare an ethanolic solution containing dioctyl sulfosuccinate sodium and ethyl cellulose, wherein ethyl cellulose The percentage of the total mass of the solution is 10%, and the percentage of the sodium dioctyl sulfosuccinate to the total mass of the solution is 0.68%;
[0048] (2) take by weighing fexofenadine hydrochloride and sucrose according to the recipe quantity, place in a wet granulator, after mixing, add above-mentioned ethyl cellulose / dioctyl sulfosuccinate sodium ethanolic solution for granulation, and make The obtained soft material is sieved and dried, and then sieved to gran...
Embodiment 2
[0051] The prescription of fexofenadine hydrochloride dry suspension is as follows in Table 2:
[0052] Table 2 embodiment 2 fexofenadine hydrochloride dry suspension prescription
[0053] prescription composition % of mass fexofenadine hydrochloride 5.00 sucrose 93.17 Ethyl cellulose 0.50 Dioctyl Sodium Sulfosuccinate 0.04 Xanthan Gum 1.07 Colloidal silica 0.22
[0054] The preparation steps are the same as those in Example 1.
Embodiment 3
[0056] The prescription of fexofenadine hydrochloride dry suspension is as follows in Table 3:
[0057] Table 3 embodiment 2 fexofenadine hydrochloride dry suspension prescription
[0058]
[0059]
[0060] The preparation steps are the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com