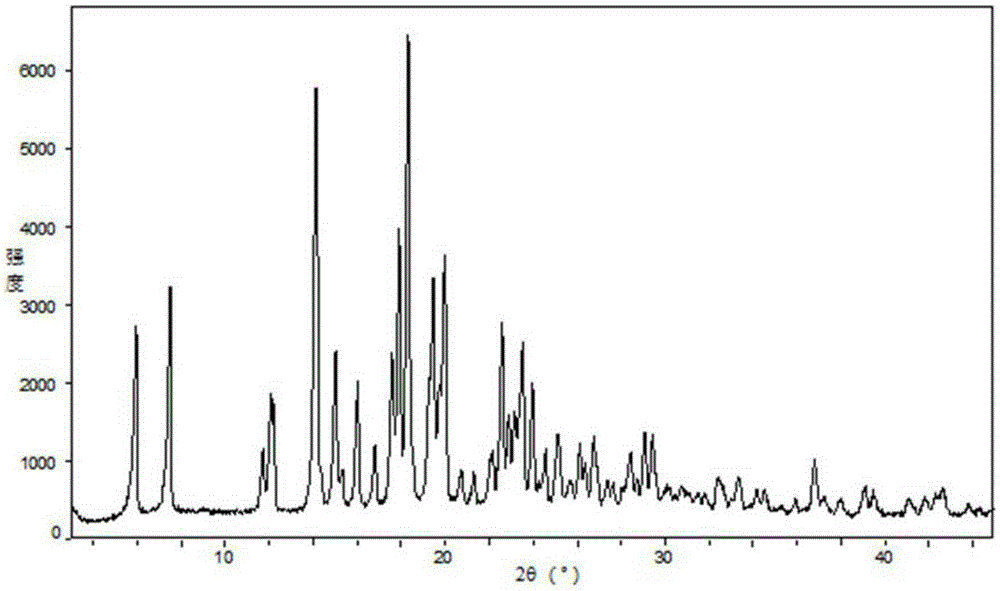
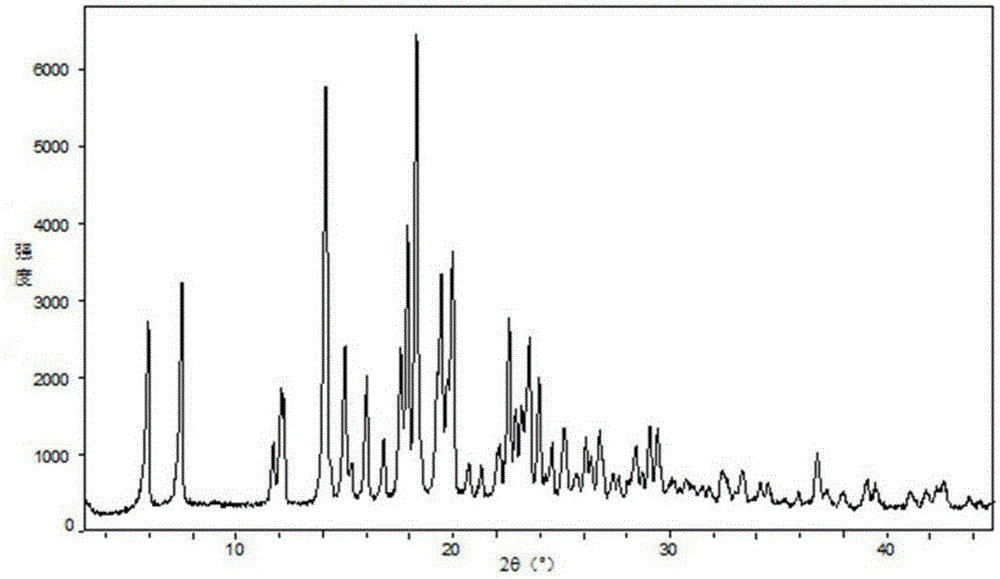
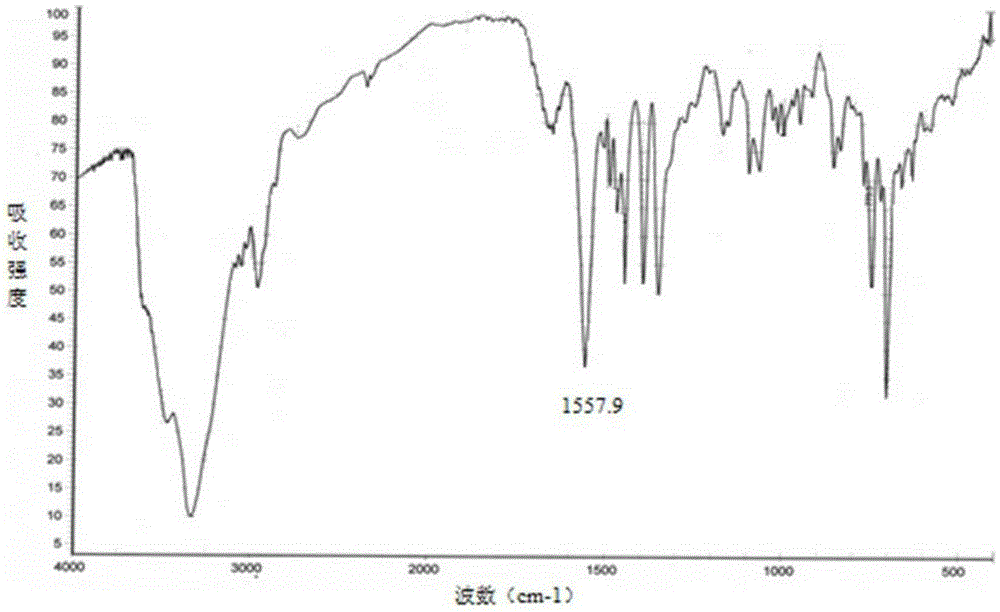
Fexofenadine hydrochloride dry suspension preparation and preparation method thereof
A technology of fexofenadine and dry suspension, applied in the field of fexofenadine hydrochloride dry suspension preparation and its preparation, can solve the problem of stability, fluidity suspension stability, dispersibility , Taste defects and other problems, to achieve the effect of good taste, easy storage and rapid absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Product prescription (Table 1)
[0032] Prescription (g) 1(% content%) Fexofenadine Hydrochloride 0.8 xanthan gum 0.76 Poloxamer 407 0.15 Disodium hydrogen phosphate dodecahydrate 3.52 Sodium dihydrogen phosphate monohydrate 2.23 blueberry flavor 0.55 Sour Strawberry Flavor 0.55 Sucralose 1.2 Glyceryl behenate 0.24 sucrose 60 xylitol 30
Embodiment 2
[0034] Product prescription (Table 2)
[0035] Prescription (g) 2(% content%) Fexofenadine Hydrochloride 4.0 Hydroxyethyl cellulose 1.0
[0036] Sodium citrate 0.98 citric acid 0.52 Sweet Orange Flavor 0.1 cream flavor 0.1 saccharin 0.7 Carbomer 974 2.6 sucrose 60 xylitol 30
Embodiment 3
[0038] Product prescription (Table 3)
[0039] Prescription (g) 3(% content%) Fexofenadine Hydrochloride 2.3 Povidone 0.5 Disodium hydrogen phosphate dodecahydrate 5.76 Sodium dihydrogen phosphate monohydrate 4.24 orange flavor 1.0 coconut flavor 1.0 Acesulfame K 0.2 Carbomer 934 5.0 Micropowder silica gel 53.3 Maltitol 26.7
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com