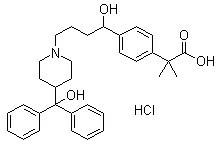
Fexofenadine hydrochloride oral disintegrating drug composition
A fexofenadine, orally disintegrating technology, applied in the field of fexofenadine hydrochloride orally disintegrating pharmaceutical composition and its preparation, can solve the problem of moisture sensitivity and easy oxidation of fexofenadine hydrochloride , low hardness, low strength, high brittleness and other problems, to achieve the effect of reducing market risk, simple method and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
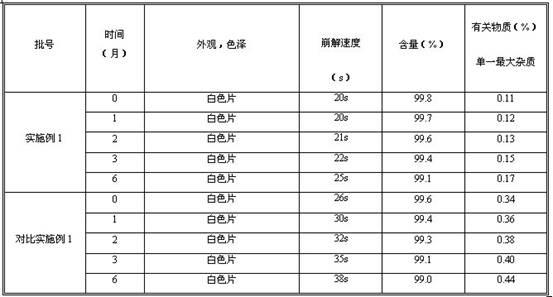
Examples
Embodiment 1
[0087] The orally disintegrating pharmaceutical composition of fexofenadine hydrochloride described in every 1000, its formula consists of:
[0088] Fexofenadine hydrochloride as fexofenadine 300g
[0089] Povidone 60g
[0090] Purified water 600g
[0091] Spray Dried Mannitol Spherical Granules 1500g
[0093] Hypromellose E5LV 300g
[0094] Purified water 2000g
[0095] Croscarmellose Sodium 300g
[0096] Aspartame 50g
[0097] Silica 10g
[0098] Fexofenadine hydrochloride orally disintegrating pharmaceutical composition of the present invention is prepared by the following method:
[0099] 1) Fexofenadine hydrochloride coating preparation:
[0100] Fexofenadine hydrochloride as fexofenadine 300g
[0101] Povidone 60g
[0102] Purified water 600g
[0103]2) Preparation of Fexofenadine Hydrochloride Granules: Put 1.5kg of spray-dried mannitol spherical particles with an average particle size of 100um into a fluidized bed coating agent, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com