Patents
Literature
36 results about "2-methylpropanoic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
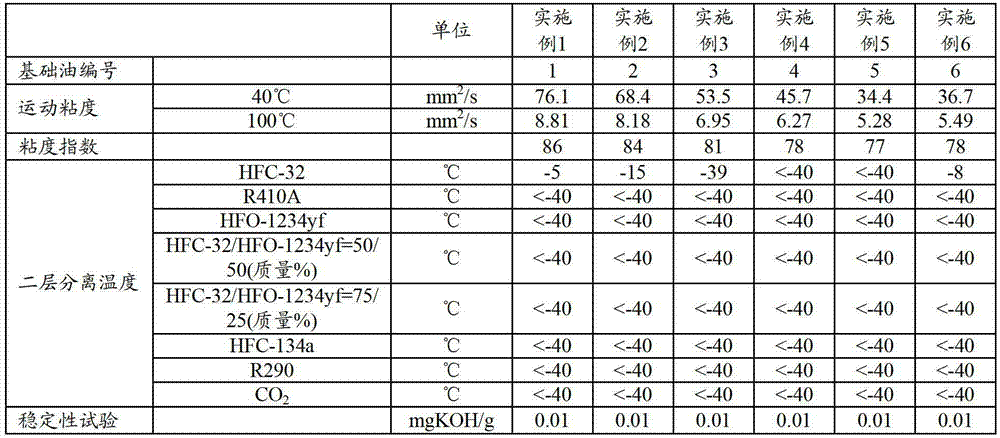
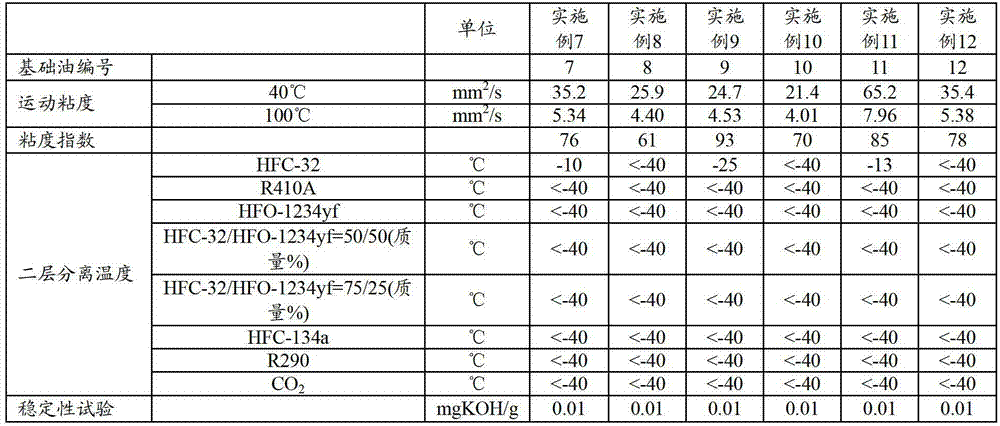
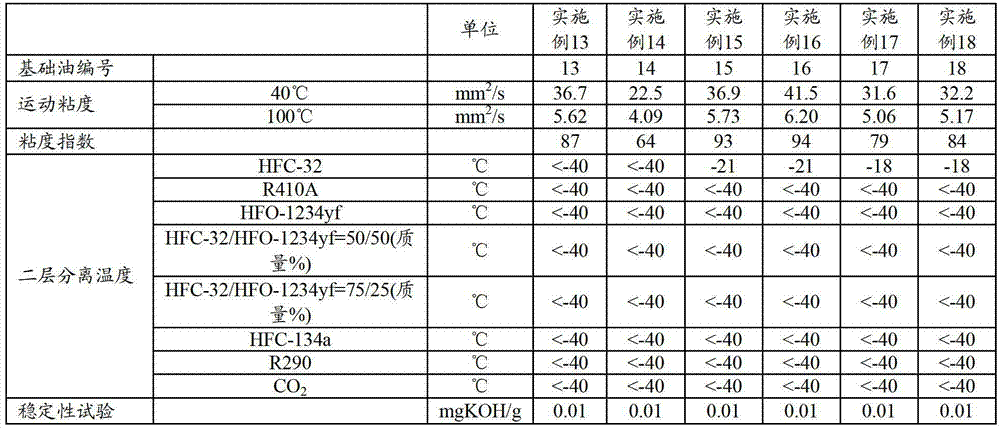
Refrigerating machine oil and working fluid composition for refrigerating machines
The refrigerating machine oil of the invention includes an ester of a polyhydric alcohol and a fatty acid, wherein the molar ratio of C4-C6 fatty acid and C7-C9 branched fatty acid in the fatty acid is between 15:85 and 90:10, the C4-C6 fatty acid includes 2-methylpropanoic acid, and the ratio of the total C4-C6 fatty acid and C7-C9 branched fatty acid in the total fatty acids composing the ester is at least 20 mol %. The working fluid composition for a refrigerating machine according to the invention comprises the refrigerating machine oil, a difluoromethane refrigerant and / or an unsaturated fluorinated hydrocarbon refrigerant.
Owner:JX NIPPON OIL & ENERGY CORP
Refrigerating machine oil and working fluid composition for refrigerating machines
ActiveCN103097501AGood compatibilityAchieve lubricationHeat-exchange elementsBase-materialsBranched chain fatty acidsWorking fluid
This refrigerating machine oil comprises esters of a polyhydric alcohol with fatty acids. In the fatty acids, the molar ratio of C4-6 fatty acids to branched C7-9 fatty acids is 15:85 to 90:10, and the C4-6 fatty acids contain 2-methylpropanoic acid, with the sum total of the C4-6 fatty acids and the branched C7-9 fatty acids accounting for at least 20 mol% of the whole of the fatty acids constituting the esters. This working fluid composition for refrigerating machines comprises the refrigerating machine oil and a difluoromethane refrigerant and / or an unsaturated fluorinated hydrocarbon refrigerant.
Owner:JX NIPPON OIL & ENERGY CORP
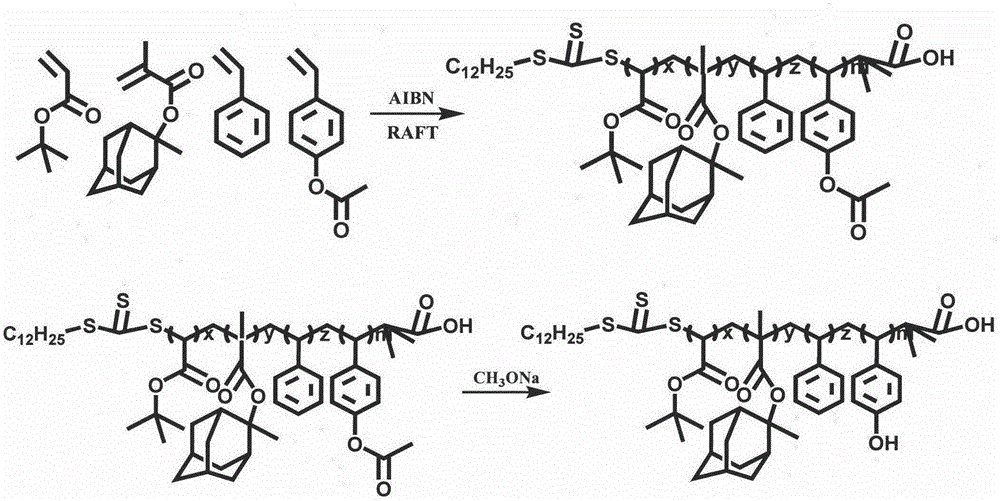
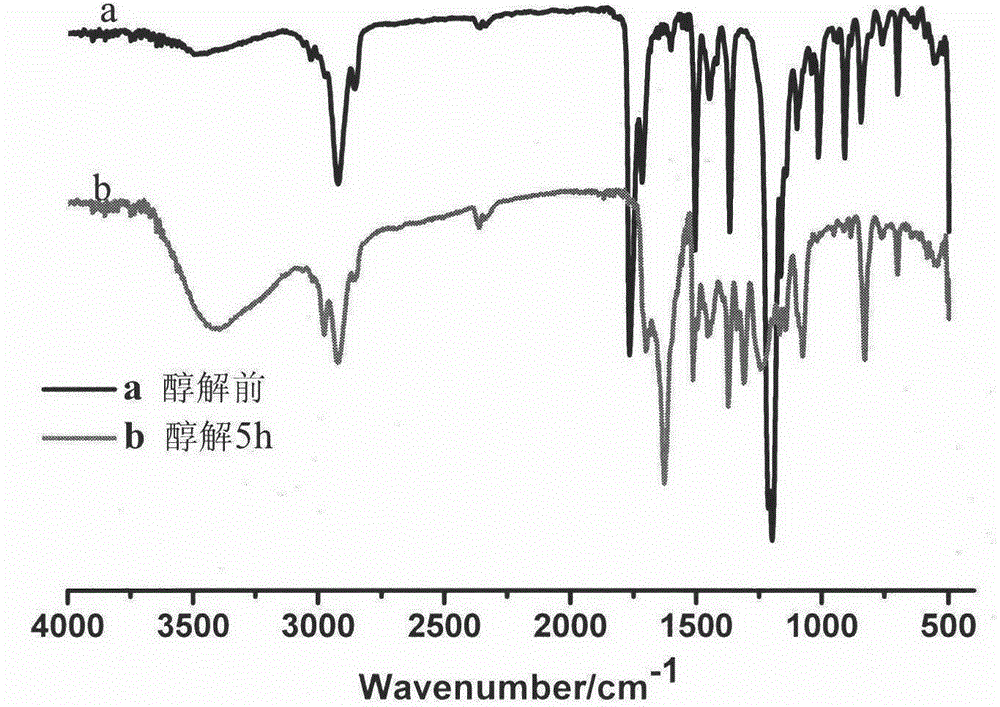
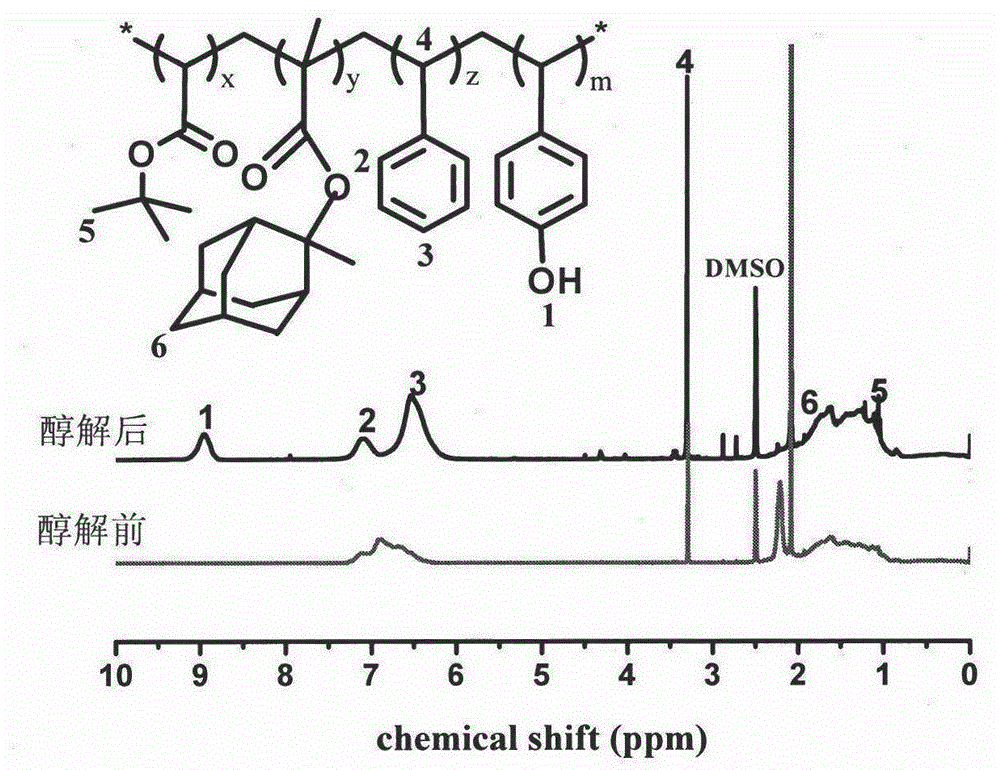
Preparation of 248 nm deep ultraviolet photoresist film forming resin based on RAFT polymerization method
ActiveCN105237669AImprove light transparencyImprove adhesionPhotosensitive materials for photomechanical apparatusSodium methoxide2-methylpropanoic acid
The present invention discloses preparation of a 248 nm deep ultraviolet photoresist film forming resin based on a RAFT polymerization method, and belongs to the field of deep ultraviolet photoresists. According to the photoresist film forming resin, mainly 2-(dodecyl trithiocarbonate-yl)-2-methylpropanoic acid is adopted as a RAFT reagent, 4-acetoxy styrene, styrene, tert-butyl acrylate and 2-methyl-2-methacrylic acid adamantine ester are adopted as copolymerization monomers, 1-methoxy-2-propyl acetate is adopted as a reaction solvent to carry out solution polymerization under the condition of a free radical initiator so as to synthesize a low molecular weight distribution copolymer, a certain amount of sodium methoxide and methanol are added to carry out alcoholysis so as to obtain the copolymer having high transparency at 248 nm, and the copolymer is finally applied in the 248 nm photoresist. According to the present invention, the preparation is simple, the reaction conditions are mild, the molecular weight distribution of the obtained copolymer is narrow, the molecular weight can be well controlled, and the resin is suitable for 248 nm deep ultraviolet exposure.
Owner:SUZHOU RUIHONG ELECTRONIC CHEM CO LTD +1
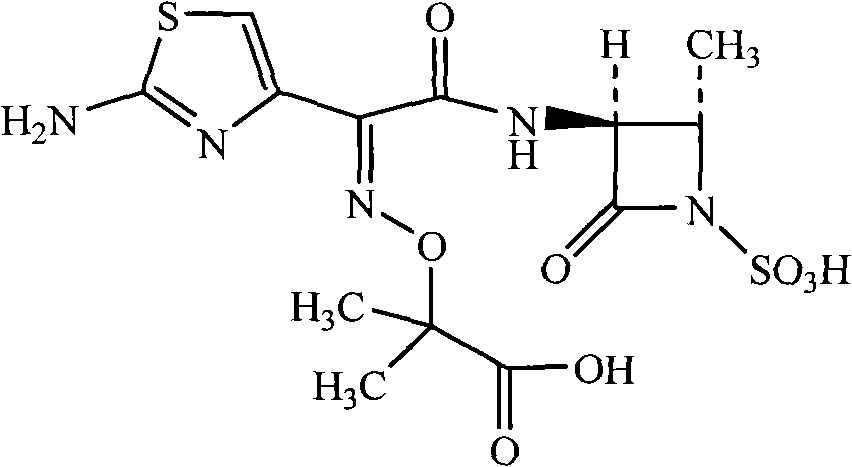
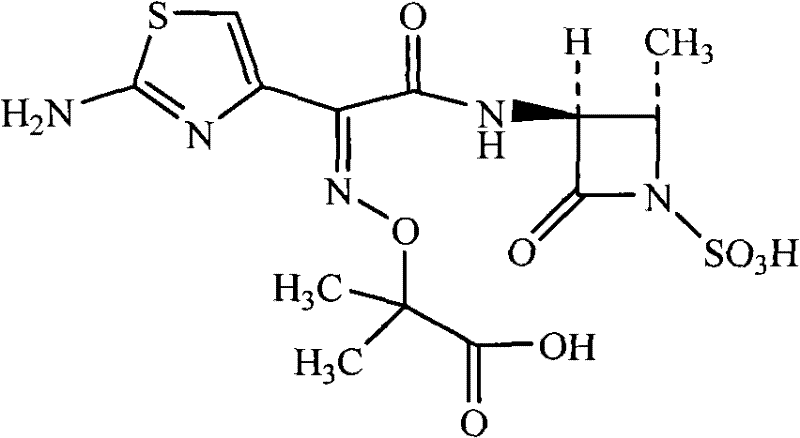
Method for synthesizing aztreonam compound
ActiveCN102127068AReduce pollutionReduce manufacturing costOrganic chemistry2-methylpropanoic acidSolvent
The invention relates to a method for synthesizing an aztreonam compound. The method comprises the following steps of: (1) adding water and a water-soluble non-aqueous solvent into a reaction container, adding trans-3(S)-amino-4-methyl-2-keto-1-azetidine sulfonic acid, triethylamine and (Z)-2-[(2-aminothiazole-4-radical)-(benzothiazole-2-sulfenyl carbonyl) methylamine oxygroup]-2-methylpropanoic acid tert-butyl ester for reacting, and adjusting the pH with acid and separating crystals out to obtain tertiary butyl aztreonam; and (2) subjecting the tertiary butyl aztreonam and acid-water mixed liquor to reaction and performing post-treatment to obtain aztreonam. By adopting the method, the reaction time can be shortened, the reaction speed can be increased, the production cost can be lowered, and environmental pollution can be reduced.
Owner:SHANXI PUDE PHARMA CO LTD
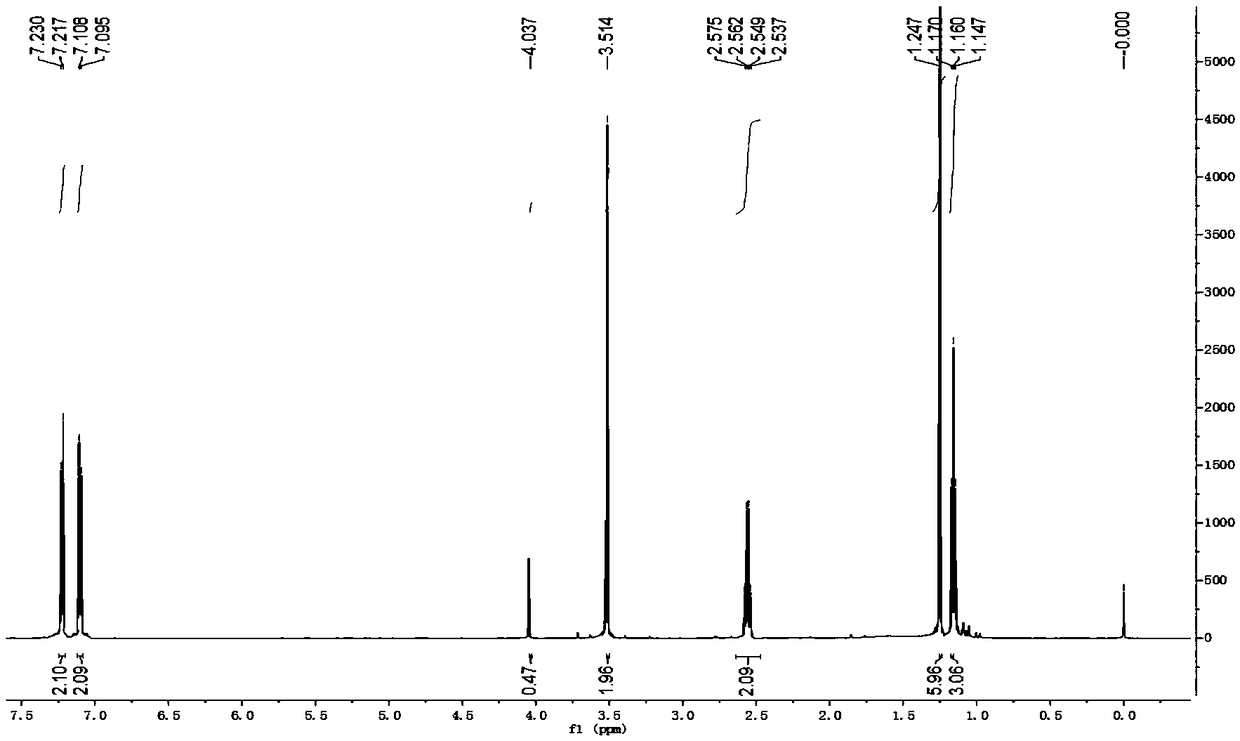
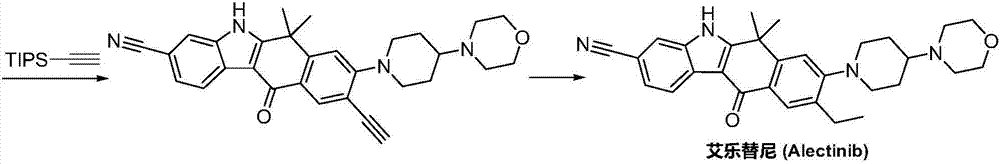
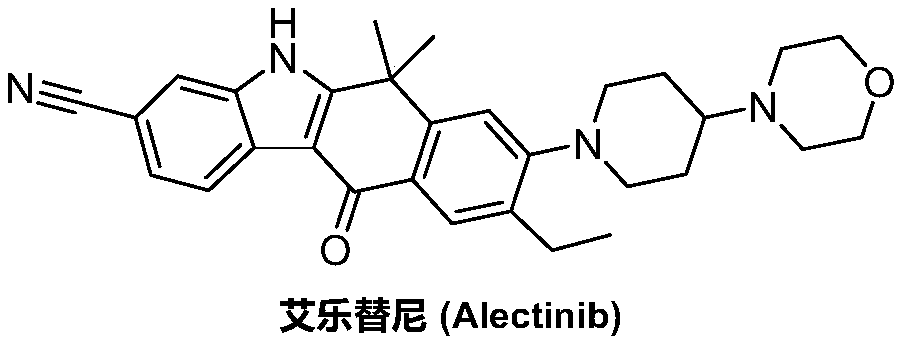
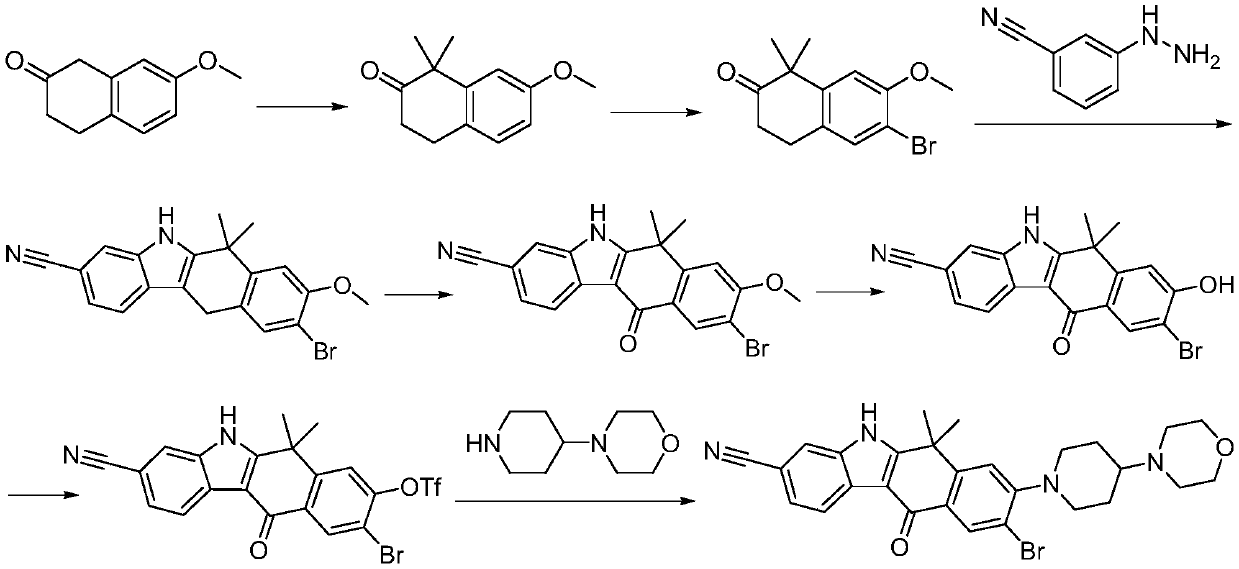
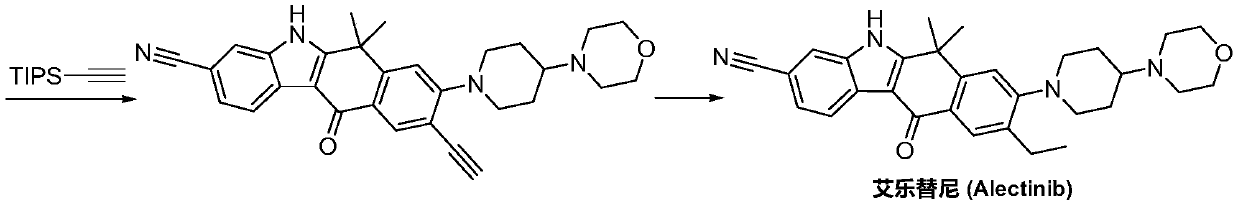
Method for synthesizing alecensa hydrochloride intermediate 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid
ActiveCN109438218AReduce usageAvoid using effectsPreparation by oxidation reactionsOrganic compound preparation2-methylpropanoic acidOxygen
The invention discloses a method for synthesizing an alecensa hydrochloride intermediate 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid. The method is characterized by comprising steps of carrying out Friedel-Crafts reaction on ethylbenzene and 3-halogen-2-methyl-1-propylene to obtain 1-halogn-2-(4-ethyl phenyl)-2-methylpropane; carrying out reaction on the 1-halogn-2-(4-ethyl phenyl)-2-methylpropane and magnesium to obtain 2-(4-ethyl phenyl)-2-methyl propyl magnesium halide; carrying out reaction on the 2-(4-ethyl phenyl)-2-methyl propyl magnesium halide and oxygen to obtain 2-(4-ethyl phenyl)-2-methyl-1-propyl alcohol; oxidizing the 2-(4-ethyl phenyl)-2-methyl-1-propyl alcohol, sodium hypochlorite and sodium chlorite by the aid of 2,2,6,6-tetramethyl piperidine oxide to obtain 2-(4-ethyl phenyl)-2-methylpropanoic acid; carrying out reaction on the 2-(4-ethyl phenyl)-2-methylpropanoic acid and iodine in the presence of oxidizing agents under acidic conditions to obtain the alecensa hydrochloride intermediate. 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid. The 2,2,6,6-tetramethyl piperidine oxide is used as a catalyst. The method has the advantages that the method is easy and convenient to operate, high in yield, low in cost and little in pollution, raw materials for the alecensa hydrochloride intermediate 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid are inexpensive and are easily available, and industrial production can be facilitated.
Owner:成都艾必克医药科技有限公司
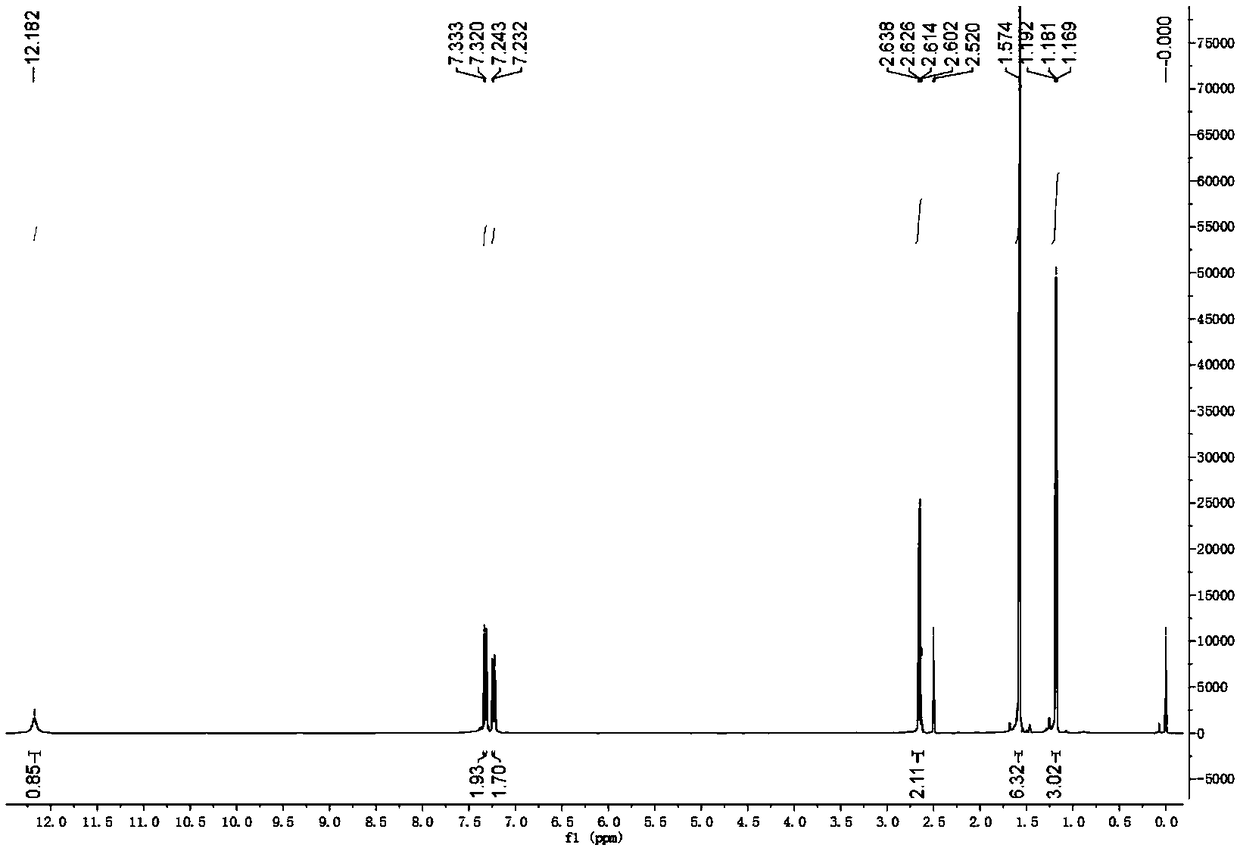
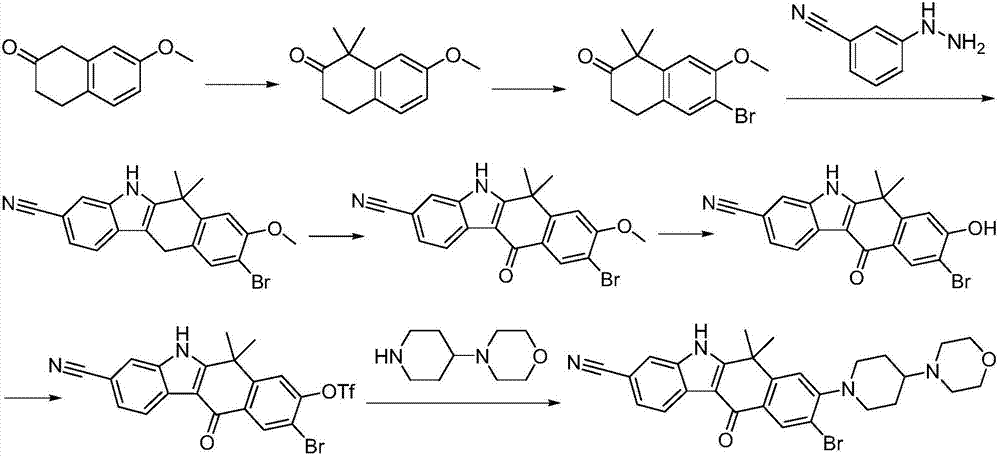
Preparation method of alectinib intermediate
ActiveCN106892860AMeet the needs of useUse requirements applyOrganic chemistryMorpholineTriflic acid
The invention discloses a preparation method of an alectinib intermediate, tert-butyl 4-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}-4-methyl-3-oxovalerate. The preparation method comprises: subjecting ethyl 2-(4-ethyl-3-hydroxyphenyl)acetate and triflic anhydride to triflic acid esterification; subjecting the obtained 5-[(ethoxycarbonyl)methyl]-2-ethylphenyltriflate and 4-(4-piperidyl)morpholine to substitution reaction; subjecting the obtained ethyl 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}acetate and methyl iodide to bis-methylation reaction; hydrolyzing the obtained ethyl 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}-2-methylpropanoate; subjecting the obtained 2-{4-ethyl-3-[4-(morpholine-4-yl)piperidine-1-yl]phenyl}-2-methylpropanoic acid and mono-tert-butyl malonate to condensation reaction to obtain the alectinib intermediate. The preparation method is simple to perform and low in cost, is a green technique and is applicable to industrial production.
Owner:湖南润星制药有限公司
Method for synthesizing 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid
InactiveCN101585762AFew reaction stepsSimple preparation processPreparation from carboxylic acid amidesAlcoholMethylene Dichloride
The invention discloses a method for synthesizing 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping the alcohol solvent of N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide in the alcohol solvent, agitating and reacting for 10-30h at 20-50 DEG C, extracting, drying and filtering to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in the alcohol solvent of the alkali metal hydroxide, refluxing and reacting for 20-40h, and adjusting the pH of the reaction mixture to be 3 by hydrochloric acid, extracting, drying, filtering and removing methylene dichloride to obtain 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid. The synthetic method of the invention has the advantages of a few reaction steps, simple preparation technology, high yield, little pollution and lowproduction cost.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
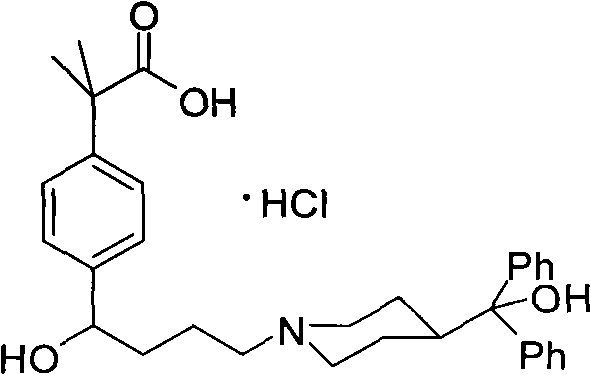
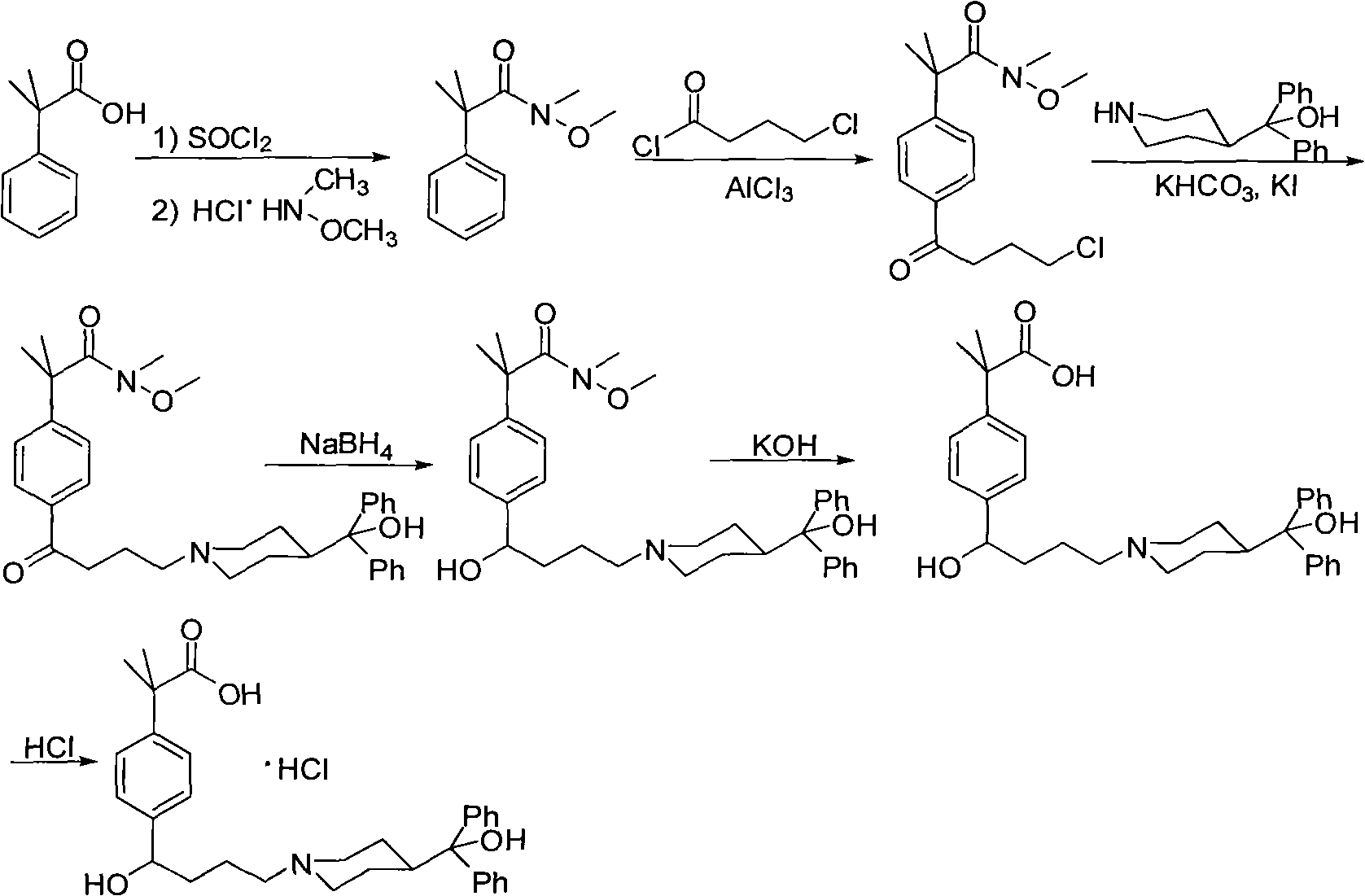
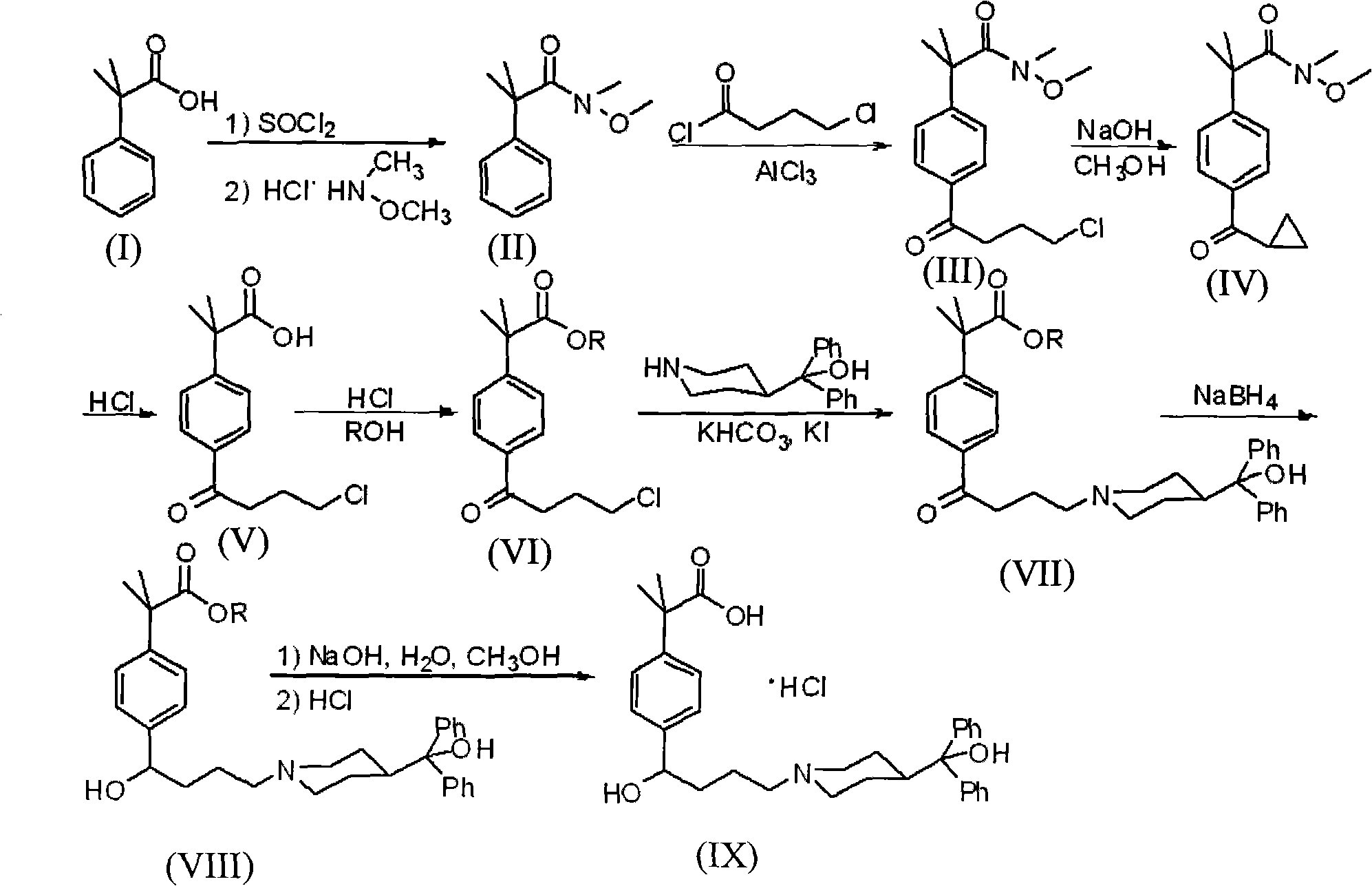
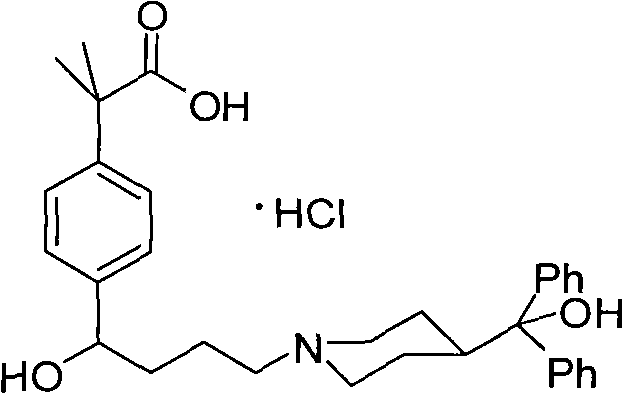
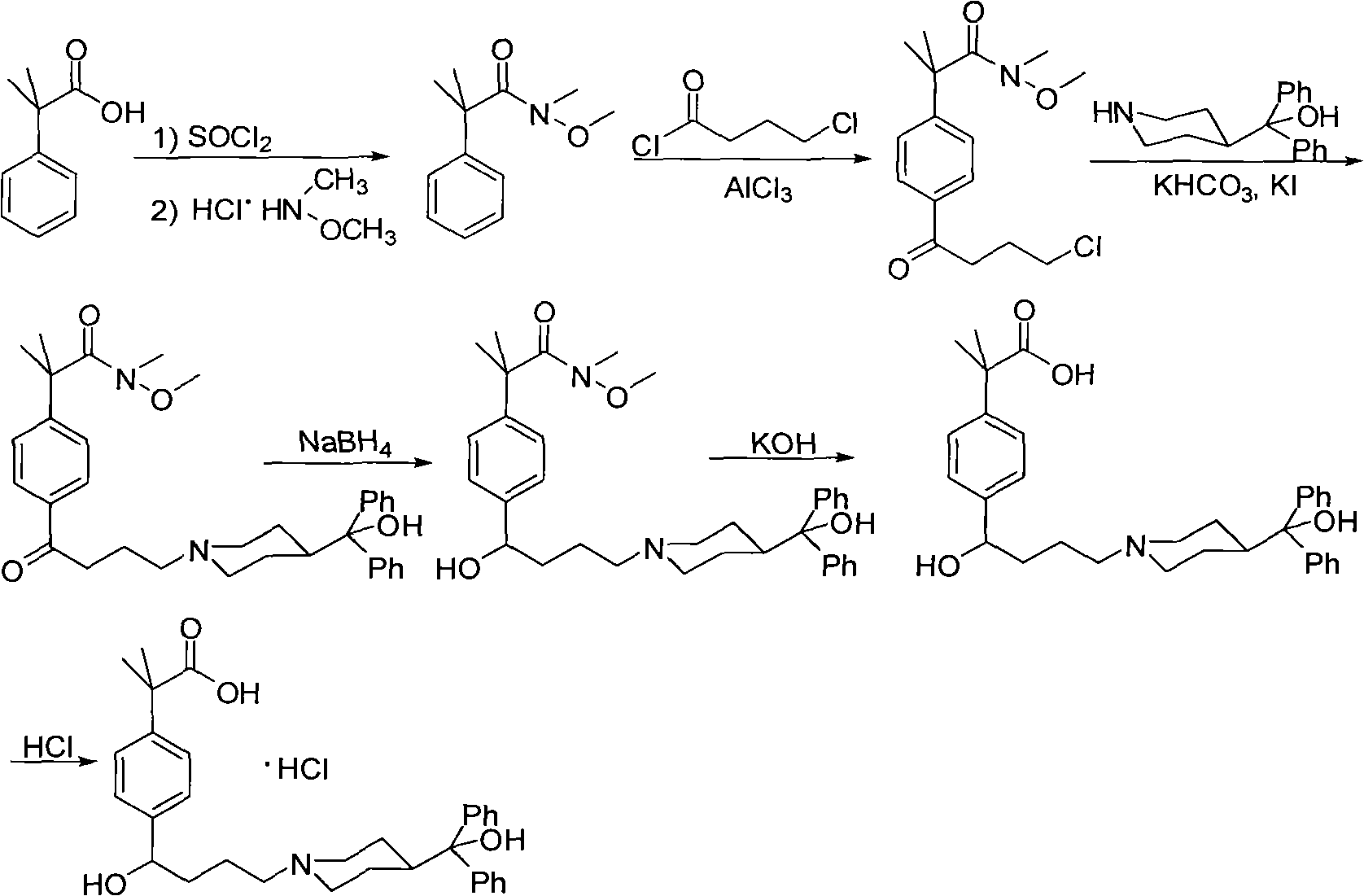
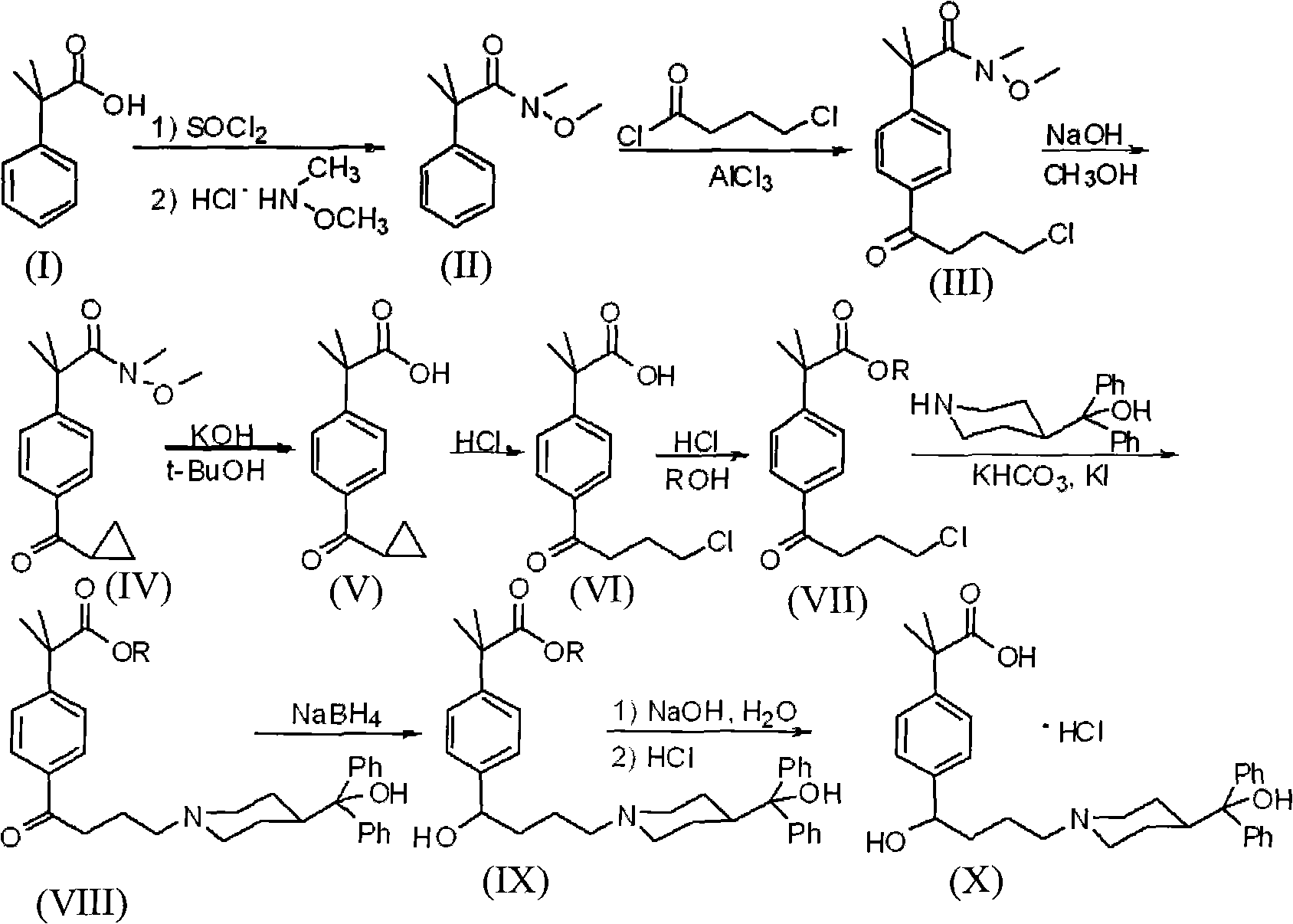
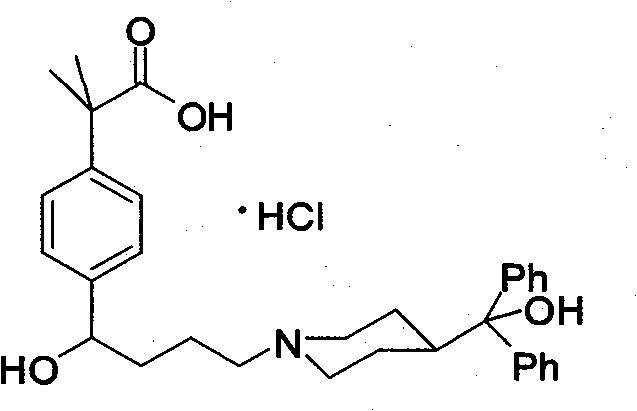
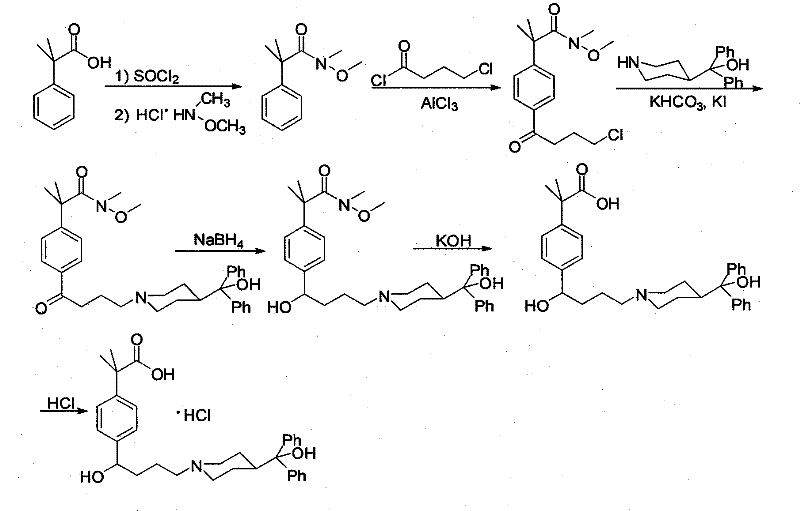
Preparation method of an antiallergic agent fexofenadine hydrochloride
InactiveCN101585805AHigh yieldReduce pollutionOrganic chemistryImmunological disordersMethacrylate2-methylpropanoic acid
The invention discloses a preparation method of an antiallergic agent fexofenadine hydrochloride, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting for 10-30h at 20-50 DEG C, conventionally processing to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an inorganic acid, reacting for 20-30h at 60 DEG C, conventionally processing and crystallizing by ethanol to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid in the alcohol solvent in which HCl gas is infused, conventionally processing to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, and finally obtaining the target product of the invention through routine techniques. The invention has the advantages of high yield, freeness of meta-isomers and amide impurities, little pollution and applicable industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
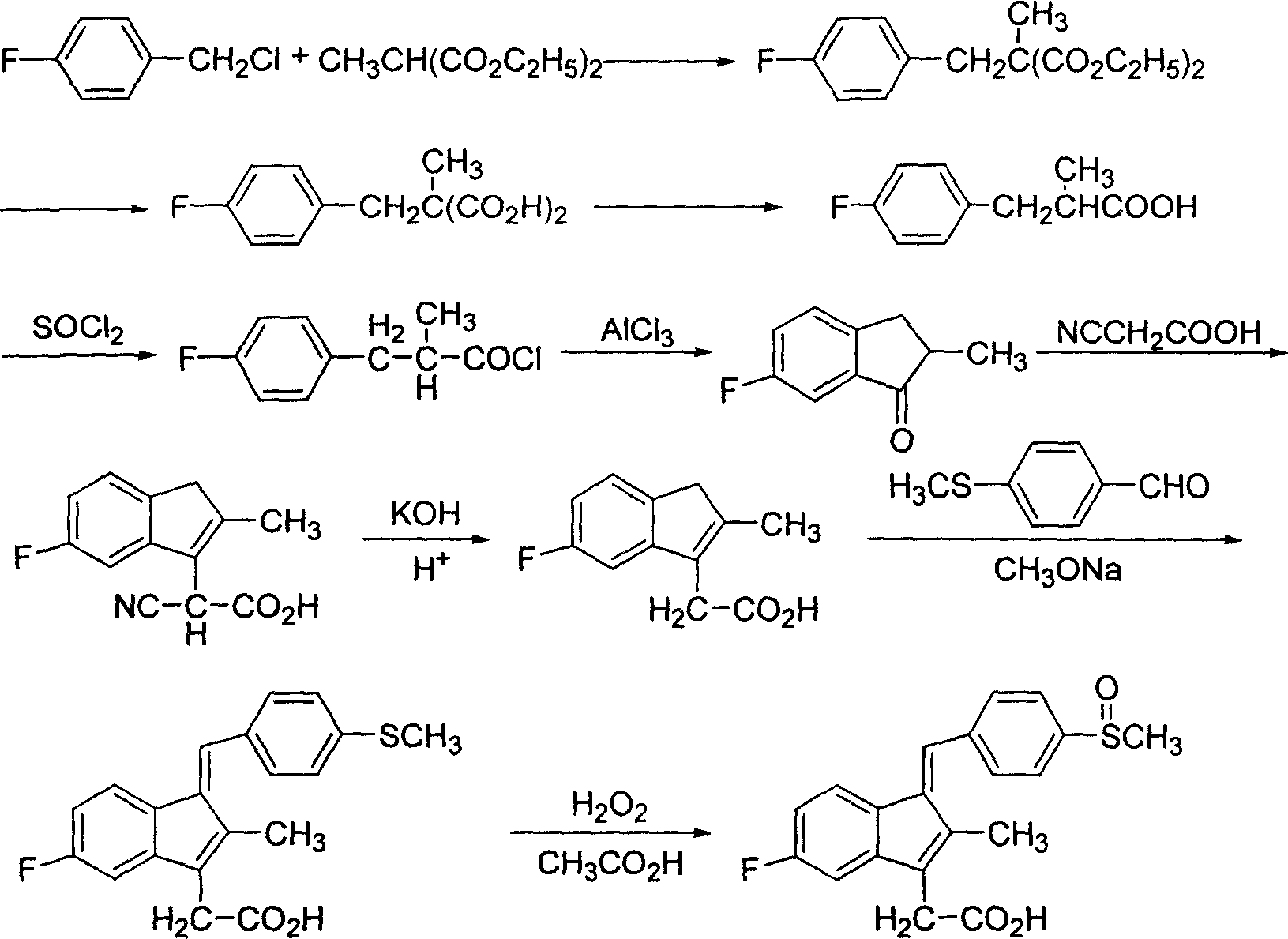
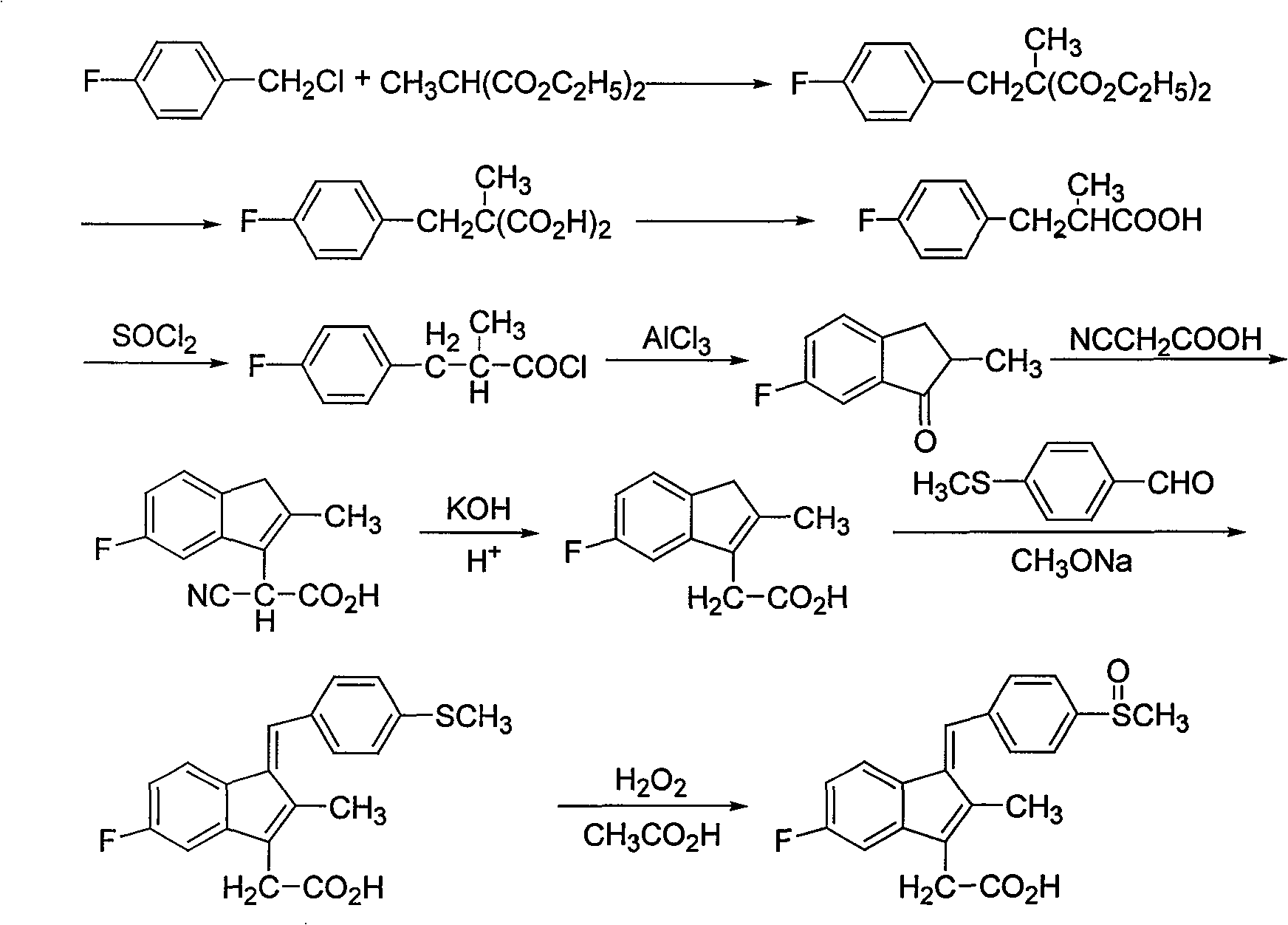
Process for preparing sulindac
InactiveCN1699335AReduce productionHigh yieldOrganic chemistryOrganic compound preparationMethyl malonic acidCyanoacetic acid
The invention discloses a process for preparing sulindac comprising the steps of, condensating 4-fluorobenzyl chloride and diethyl methylmalonate at the presence of organic base, obtaining 2-(4-fluorobenzyl)-2-diethyl methylmalonate, hydrolyzing in aqueous alkali to obtain 2-(4-fluorobenzyl)-2-methyl malonic acid, carrying out decarboxylation directly under high temperature to obtain 3-(4-fluorophenyl)-2-methylpropanoic acid, chlorinating with sulfoxide acyl chloride, then using aluminium trichloride or waterless zinc chloride as catalyst, carrying out F-C acylation to obtain 6-fluoro-2-methylindanone, condensing with cyanocetic acid, hydrolyzing to obtain 5-fluoro-methyl-3-indenes acetic acid, then condensing with p-methylthiobenzaldehyde to obtain 5-fluoro-2-methyl-1-(4-methylthiobenzal)-3-indenes acetic acid, finally using organic acid as solvent, peracid or hydroperoxide as oxidation agent to obtain the sulindac.
Owner:ZHEJIANG UNIV
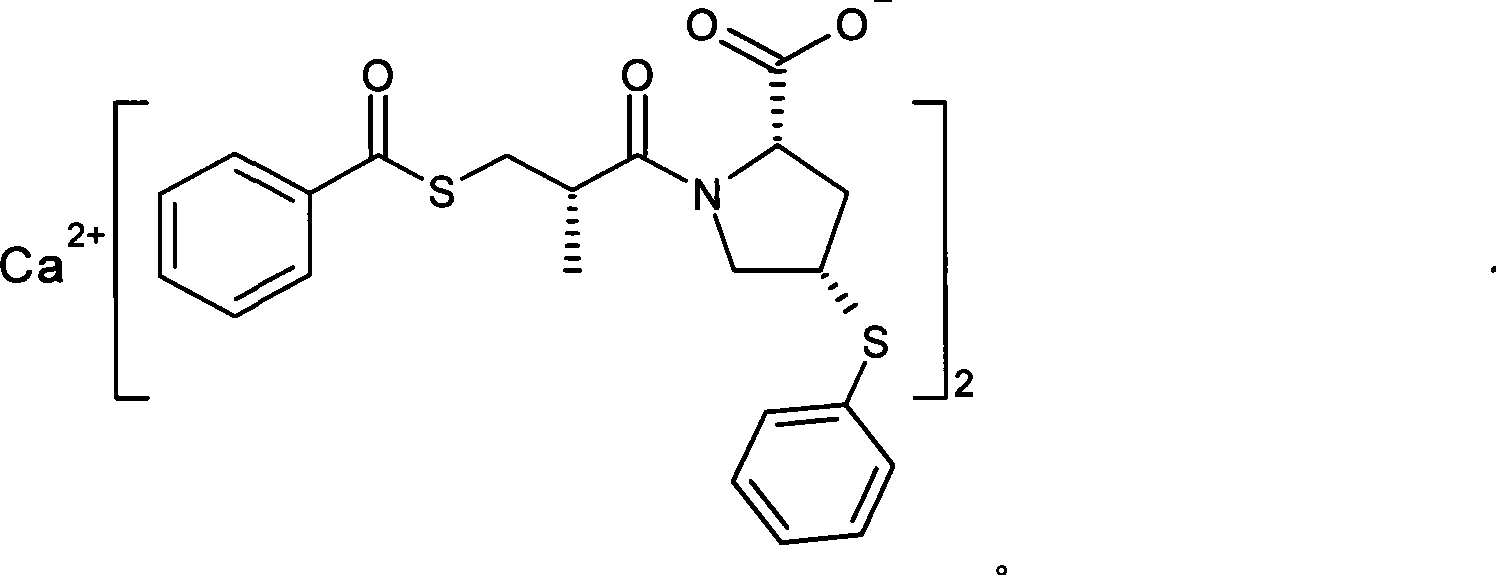
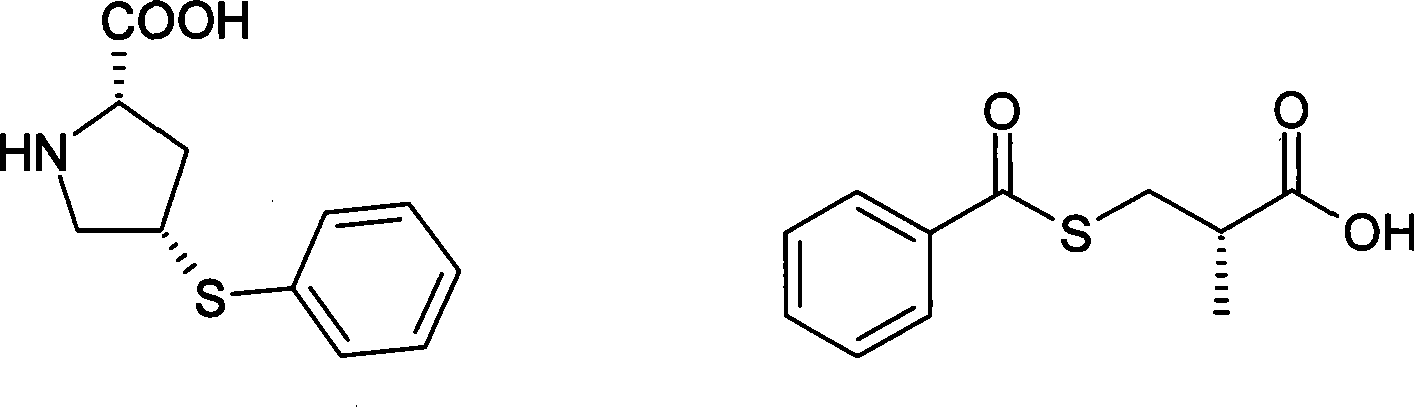
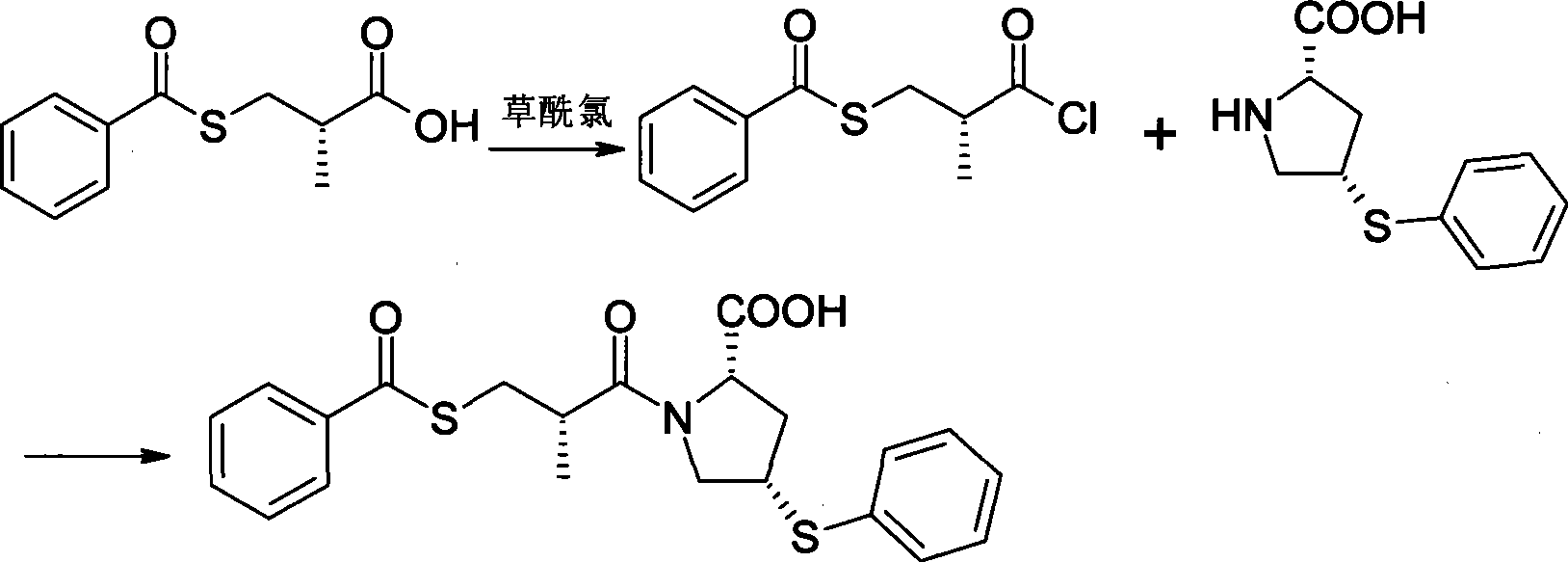
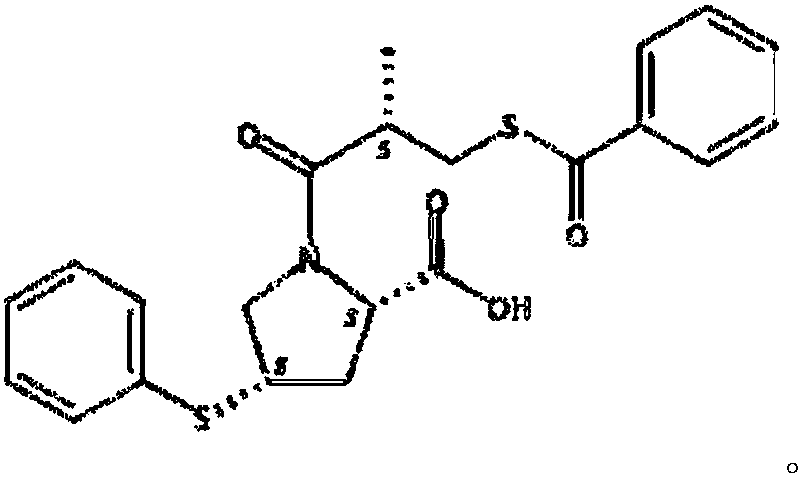
Method for preparing zofenopril calcium
InactiveCN103936644AEliminate effectiveEffective controlOrganic chemistry2-methylpropanoic acidCalcium EDTA
The invention provides a method for preparing zofenopril calcium. N-acetyl-L-oxyproline is adopted as a raw material. The method includes: a step of subjecting the N-acetyl-L-oxyproline and methanol to esterification, subjecting the obtained product and paratoluensulfonyl chloride to sulfonation, and subjecting the obtained product and thiophenol prepared from a sodium thiophenolate solution to thiophenyl substitution; a step of hydrolyzing the obtained product into an free acid by utilization of an alkali, performing recrystallization for purification, and performing deacetylation with hydrochloric acid to obtain (cis)-4-thiophenyl-L-proline hydrochloride; a step of reacting (S)-3-(benzoyl sulfhydryl)-2-methylpropanoic acid with thionyl chloride to obtain (S)-3-(benzoyl sulfhydryl)-2-methyl propionylchloride; a step of reacting the (S)-3-(benzoyl sulfhydryl)-2-methyl propionylchloride with the (cis)-4-thiophenyl-L-proline hydrochloride to obtain the free acid zofenopril; and a step of forming a potassium salt, purifying and reacting with a calcium chloride to obtain a calcium salt, thus obtaining a final product zofenopril calcium. The method has characteristics of easily available raw materials, simple preparation method, mild conditions, easy control, reasonable monitoring points in the preparation process, effective removal and control of impurities, capability of producing the final product with the needed crystal form preferentially in a high ratio, suitability for industrial production and large application value.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
Method for synthesizing Zofenopril
InactiveCN101372472ASimple process routeMild conditionsOrganic chemistryHydroxyproline2-methylpropanoic acid
The invention provides a method for synthetizing Zofenopril Calcium salt, which takes N-acetyl-L-oxyproline as raw materials; the N-acetyl-L-oxyproline and methanol are esterified, paratoluensulfonyl chloride is sulphonated, and thiophenyl is substituted; the obtained product is hydrolyzed to be freeacid by alkali, and (cis form)-4-thiophenyl-L-proline hydrochloride is further obtained by hydrochloric acid deacetylation reaction; in addition, (S)-3-(benzoyl sulfhydryl group)-2-methylpropanoic acid reacts with thionyl chloride, and (S)-3-(benzoyl sulfhydryl group)-2-methyl propionyl chloride which then reacts with the (cis form)-4-thiophenyl-L-proline hydrochloride, so that Zofenopril freeacid is obtained; the Zofenopril freeacid is firstly made into sylvite, and then refined and purified to be calcium salt, so that the termination product is obtained. The materials of the method of the invention is easily obtained, the process route is simple, and the reaction conditions are mild; therefore, the method is easy to operate and suitable for commercial process.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
A kind of preparation method of alectinib intermediate
Owner:湖南润星制药有限公司
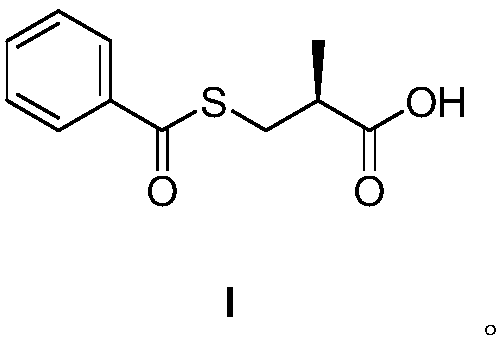
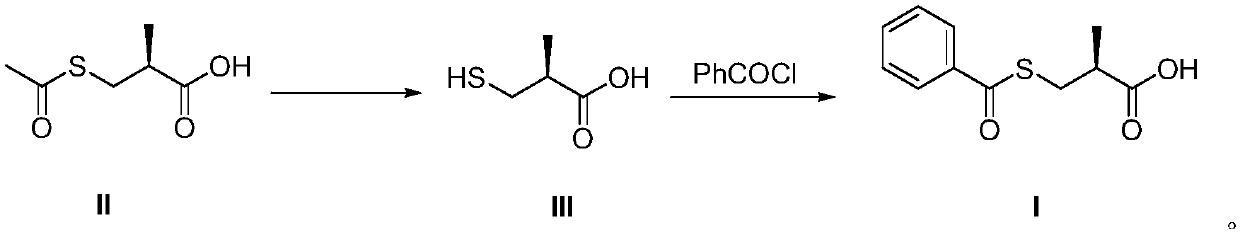
Method for preparing S-(-)-Benzoylthio-2-methylpropanoic acid compound
InactiveCN109651218AEasy to operateSave raw materialsThiol preparationOrganic chemistry methodsBenzoyl chloride2-methylpropanoic acid
The invention discloses a method for preparing a S-(-)-Benzoylthio-2-methylpropanoic acid compound. S-(-)-3-acetylthio-2-methylpropionic acid as a raw material shown in the formula II is hydrolyzed ina reacting solvent to obtain S-(-)-3-mercapto-2-methylpropionic acid shown in hte formula III, and the S-(-)-3-mercapto-2-methylpropionic acid and benzoyl chloride are subjected to condensation to obtain the S-(-)-Benzoylthio-2-methylpropanoic acid shown in the formula I. The preparation method is easy in operation, the raw materials and accessory materials are low in cost and easy to obtain, thereaction conditions are mild, the yield is high, and the quality is high. (The formula is shown in the description.).
Owner:ZHEJIANG HUAHAI PHARMA CO LTD +2
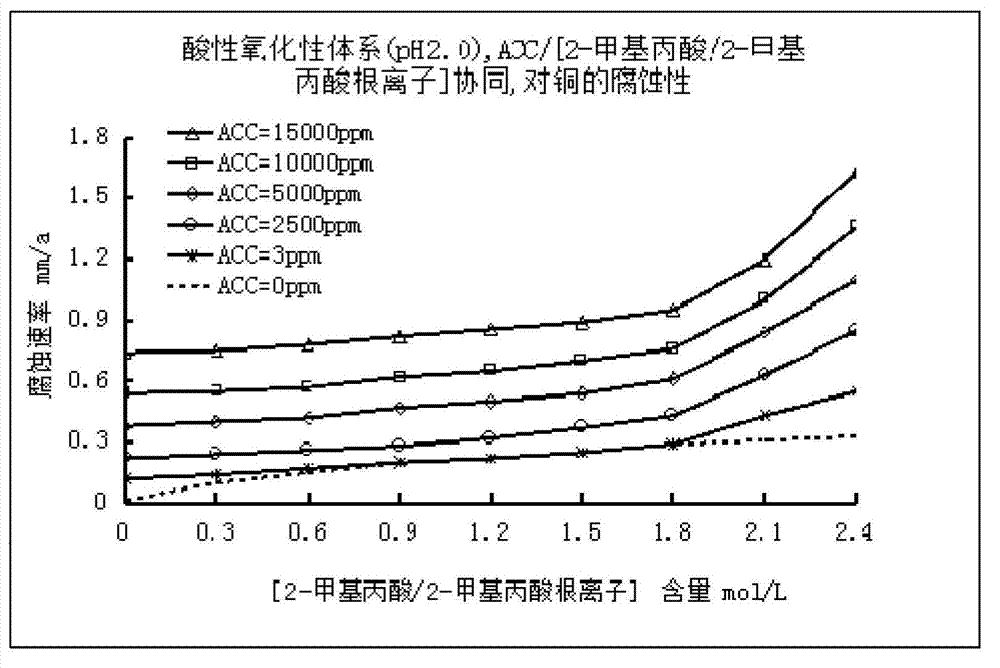
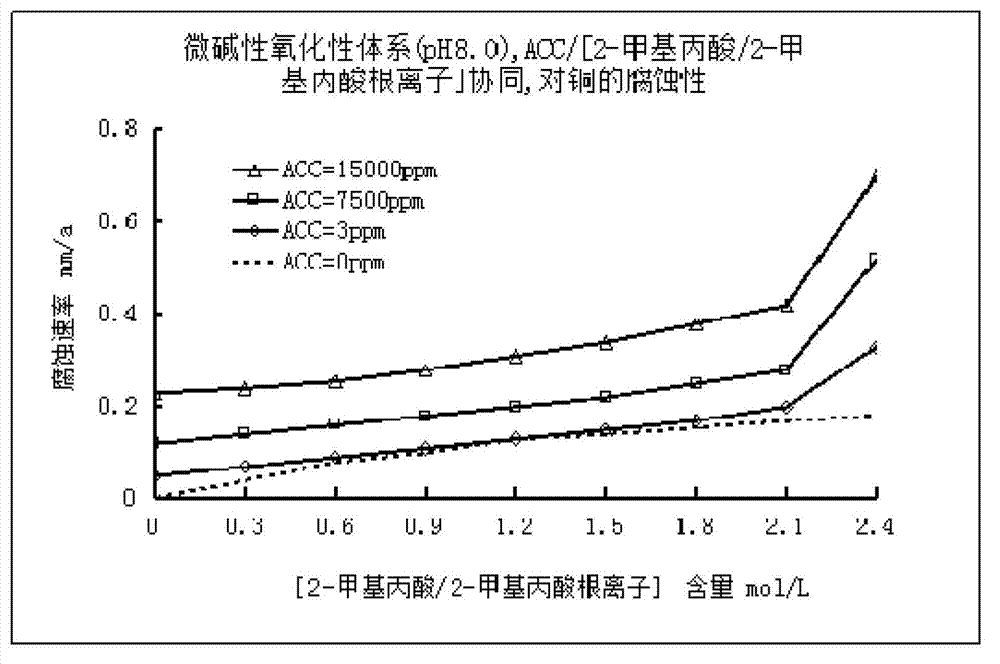
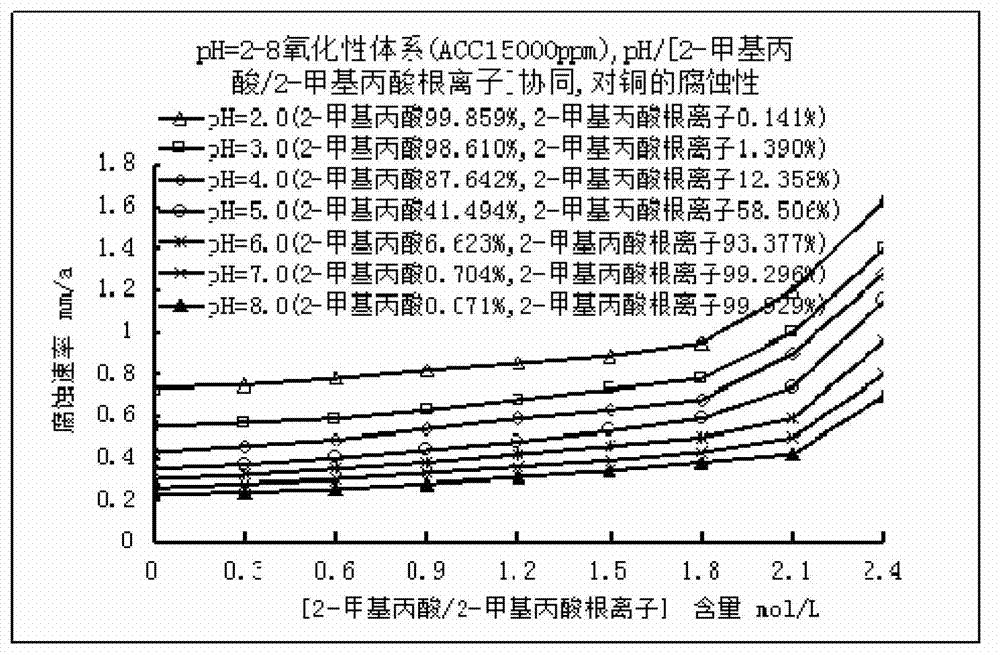
Low corrosive oxidation potential sterilization water and preparation method thereof
The invention relates to the field of sterilization and disinfection, and particularly relates to low corrosive oxidation potential sterilization water and a preparation method thereof. The preparation method of the low corrosive oxidation potential sterilization water comprises the following steps: (1) providing an available chlorine provision unit which contains available chlorine or can generate available chlorine; (2) providing a pH value regulation unit; and (3) mixing the pH value regulation unit and the available chlorine provision unit to obtain a strong oxidizing solution, wherein the pH value of the strong oxidizing solution is 2-8, the oxidation-reduction potential is not lower than 600 mV, the available chlorine content is not lower than 3 mg / L, and the sum of the 2-methylpropanoic acid content and the 2-methylpropionate ion content is not higher than 1.8 mol / L. Compared with the existing acidic oxidation potential sterilization water, the sterilization water prepared by the oxidation potential sterilization water preparation method can reduce the corrosion to metal, thereby widening the application range.
Owner:邵鹏飞
Refrigerating machine oil and working fluid composition for refrigerating machines
ActiveUS9005470B2Improve the level ofHeat-exchange elementsBase-materialsBranched chain fatty acidsPolyol
The refrigerating machine oil of the invention includes an ester of a polyhydric alcohol and a fatty acid, wherein the molar ratio of C4-C6 fatty acid and C7-C9 branched fatty acid in the fatty acid is between 15:85 and 90:10, the C4-C6 fatty acid includes 2-methylpropanoic acid, and the ratio of the total C4-C6 fatty acid and C7-C9 branched fatty acid in the total fatty acids composing the ester is at least 20 mol %. The working fluid composition for a refrigerating machine according to the invention comprises the refrigerating machine oil, a difluoromethane refrigerant and / or an unsaturated fluorinated hydrocarbon refrigerant.
Owner:JX NIPPON OIL & ENERGY CORP
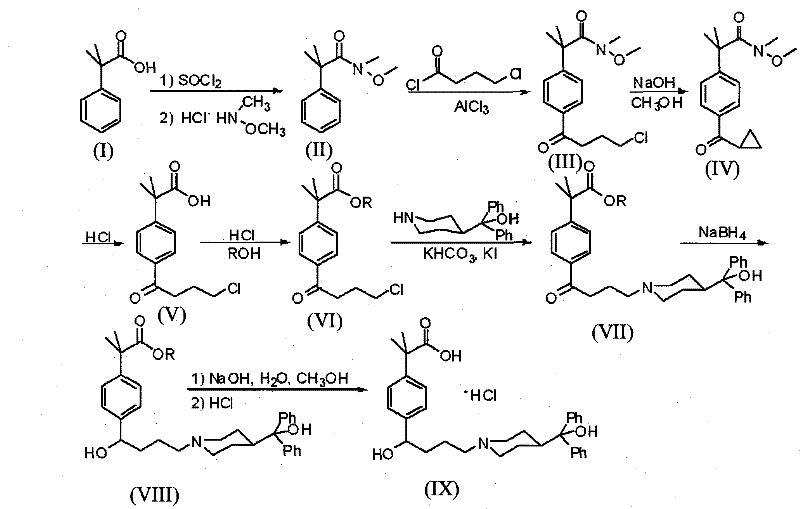
Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate
InactiveCN101585768AHigh yieldReduce pollutionOrganic compound preparationCarboxylic acid esters preparationMethacrylateAlcohol
The invention discloses a method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting for 10-30h at 20-50 DEG C, extracting, drying, and filtering to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an inorganic acid, reacting for 20-30h at 60 DEG C, extracting, drying, filtering, crystallizing by ethanol to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; infusing HCl gas in the alcohol solvent, then adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid, washing by water, drying and filtering to obtain the target product. The synthetic method of the invention has high yield, little pollution and applicable industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for synthesizing aztreonam compound
ActiveCN102127068BReduce pollutionReduce manufacturing costOrganic chemistry2-methylpropanoic acidSolvent
The invention relates to a method for synthesizing an aztreonam compound. The method comprises the following steps of: (1) adding water and a water-soluble non-aqueous solvent into a reaction container, adding trans-3(S)-amino-4-methyl-2-keto-1-azetidine sulfonic acid, triethylamine and (Z)-2-[(2-aminothiazole-4-radical)-(benzothiazole-2-sulfenyl carbonyl) methylamine oxygroup]-2-methylpropanoic acid tert-butyl ester for reacting, and adjusting the pH with acid and separating crystals out to obtain tertiary butyl aztreonam; and (2) subjecting the tertiary butyl aztreonam and acid-water mixed liquor to reaction and performing post-treatment to obtain aztreonam. By adopting the method, the reaction time can be shortened, the reaction speed can be increased, the production cost can be lowered, and environmental pollution can be reduced.
Owner:SHANXI PUDE PHARMA CO LTD
Synthetic method of a fexofenadine hydrochloride
InactiveCN101585804AHigh yieldReduce pollutionOrganic chemistryImmunological disordersMethacrylate2-methylpropanoic acid
The invention discloses a synthetic method of a fexofenadine hydrochloride, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; refluxing the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an alkaline alcohol solvent and adjusting pH to obtain 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid; reacting the 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid in HCl for 20-30h at 60-100 DEG C and recrystallizing to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid in the hydrochloric acid solution of absolute alcohol to prepare the 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, and finally obtaining the target product of the invention through routine techniques. The invention has the advantages of high yield, freeness of meta-isomers and amide impurities, little pollution and applicable industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
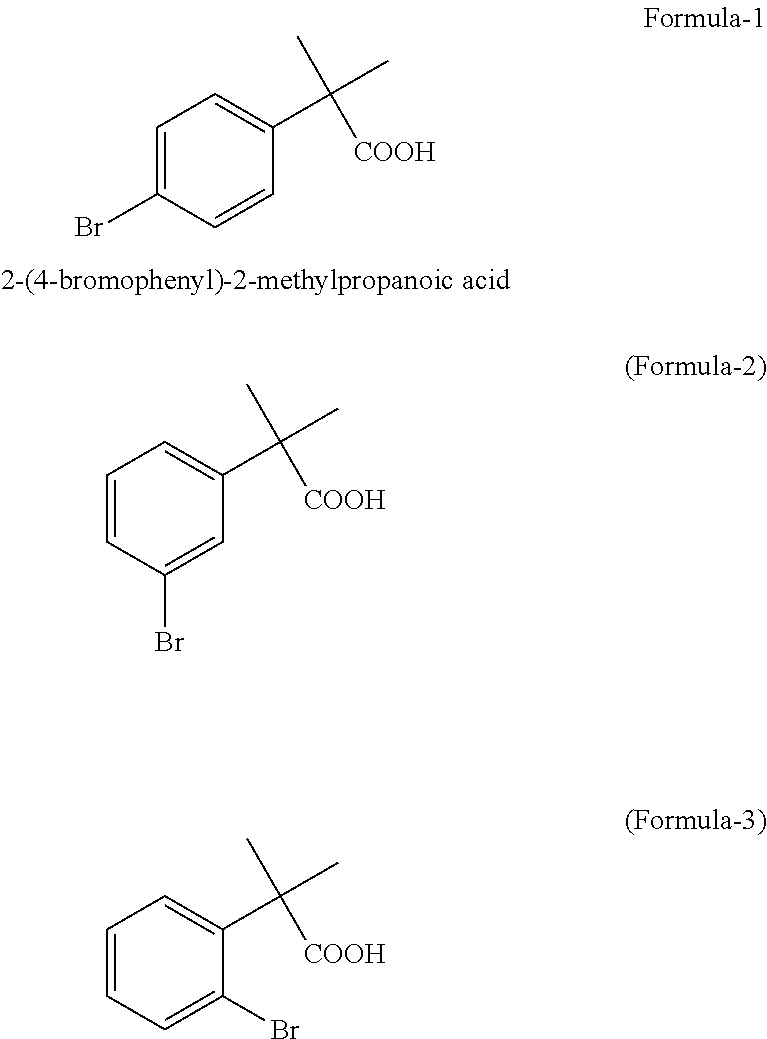
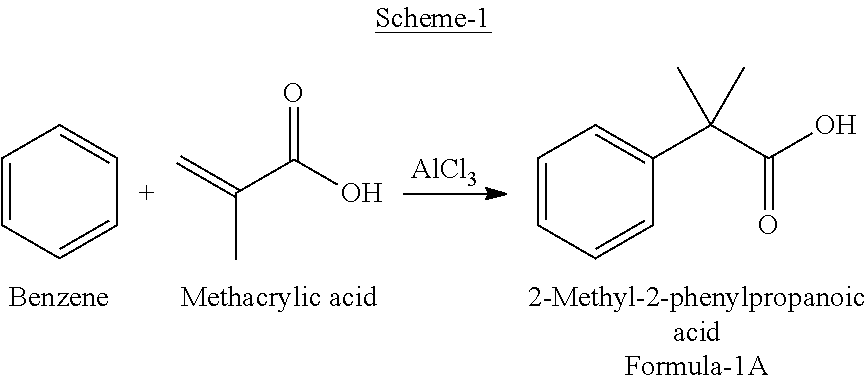
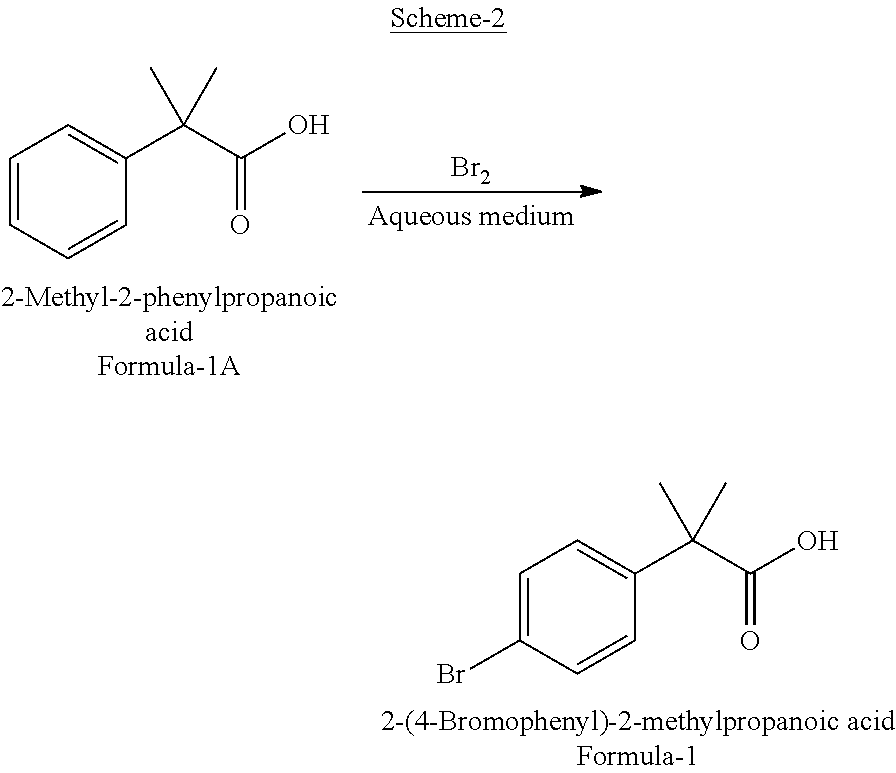
Preparation of 2-(4-bromophenyl)-2-methylpropanoic acid
InactiveUS20120309973A1Organic compound preparationCarboxylic compound preparation3-phenylpropanoic acidFexofenadine
Selective bromination of 2-methyl-2-phenylpropanoic acid in aqueous medium is described to obtain pure 2-(4-bromophenyl)-2-methylpropanoic acid, which is a useful key intermediate in the process of manufacturing pure fexofenadine.
Owner:DIVI S LAB LTD
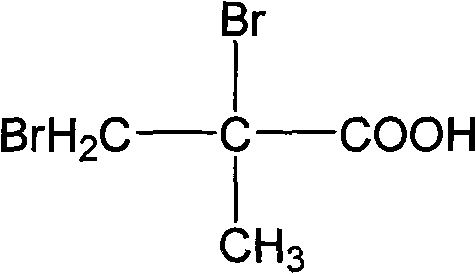
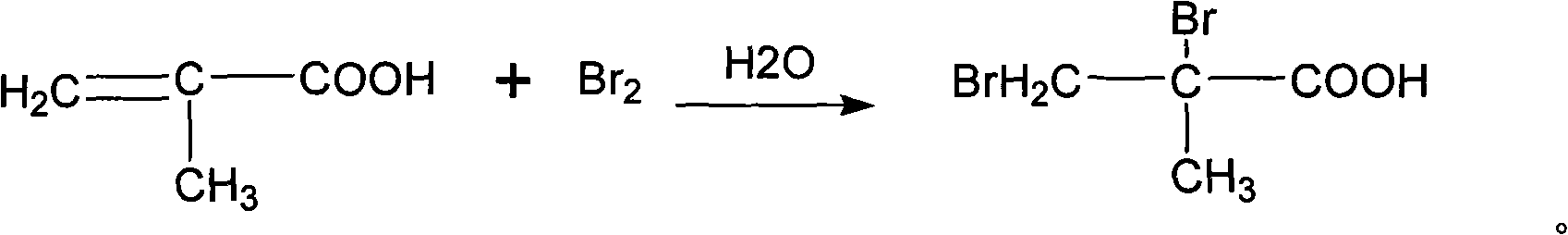
Synthetic method of 2, 3-dibromo-2-methylpropanoic acid
InactiveCN101560147AAvoid harmEasy to operateOrganic compound preparationCarboxylic compound preparationFiltrationBromine
The invention discloses a synthetic method of 2, 3-dibromo-2-methylpropanoic acid, which takes methacrylic acid and bromine as raw materials and comprises the following steps of: 1) adding bromine into methacrylic acid water solution, reacting for 1h to 5h at the temperature of 50 to 100 DEG C, and obtaining crude product solution of 2, 3-dibromo-2-methylpropanoic acid; wherein the mol ratio of methacrylic acid and bromine is 1: 1 to 1: 3; 2) obtaining mother liquid and solid by carrying out temperature reduction and crystallization, and filtration; and 3) obtaining the 2, 3-dibromo-2-methylpropanoic acid by washing the solid for 2 to 3 times, and drying. The method for producing 2, 3-dibromo-2-methylpropanoic acid has the advantages of high yield coefficient, environmental protection and the like.
Owner:ZHEJIANG UNIV
Preparation method of an antiallergic agent fexofenadine hydrochloride
InactiveCN101585805BHigh yieldReduce pollutionOrganic chemistryImmunological disordersMethacrylate2-methylpropanoic acid
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate
InactiveCN101585768BHigh yieldReduce pollutionOrganic compound preparationCarboxylic acid esters preparationMethacrylateAlcohol
The invention discloses a method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting for 10-30h at 20-50 DEG C, extracting, drying, and filtering to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an inorganic acid, reacting for 20-30h at 60 DEG C, extracting, drying, filtering, crystallizing by ethanol to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; infusing HCl gas in the alcohol solvent, then adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid, washing by water, drying and filtering to obtain the target product. The synthetic method of the invention has high yield, little pollution and applicable industrial production.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate
InactiveCN101585767AHigh yieldReduce pollutionOrganic compound preparationCarboxylic acid esters preparationMethacrylateAlcohol
The invention discloses a method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide alcohol solvent in the solvent, reacting for 10-30h at 20-50 DEG C to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; refluxing the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an alkaline alcohol solvent for 20-40h and adjusting pH to be 3 so as to obtain 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid; reacting the 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid in an inorganic acid for 20-30h at 60-100 DEG C and recrystallizing to obtain 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid; and finally adding the 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid in the hydrochloric acid solution of absolute alcohol, and reacting for 3h at 60 DEG C to obtain the target product. The preparation method has high product yield, little pollution and applicable industrial product.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Process for preparing sulindac
InactiveCN100412057CReduce productionHigh yieldOrganic chemistryOrganic compound preparationMethyl malonic acidCyanoacetic acid
The invention discloses a process for preparing sulindac comprising the steps of, condensating 4-fluorobenzyl chloride and diethyl methylmalonate at the presence of organic base, obtaining 2-(4-fluorobenzyl)-2-diethyl methylmalonate, hydrolyzing in aqueous alkali to obtain 2-(4-fluorobenzyl)-2-methyl malonic acid, carrying out decarboxylation directly under high temperature to obtain 3-(4-fluorophenyl)-2-methylpropanoic acid, chlorinating with sulfoxide acyl chloride, then using aluminium trichloride or waterless zinc chloride as catalyst, carrying out F-C acylation to obtain 6-fluoro-2-methylindanone, condensing with cyanocetic acid, hydrolyzing to obtain 5-fluoro-methyl-3-indenes acetic acid, then condensing with p-methylthiobenzaldehyde to obtain 5-fluoro-2-methyl-1-(4-methylthiobenzal)-3-indenes acetic acid, finally using organic acid as solvent, peracid or hydroperoxide as oxidation agent to obtain the sulindac.
Owner:ZHEJIANG UNIV
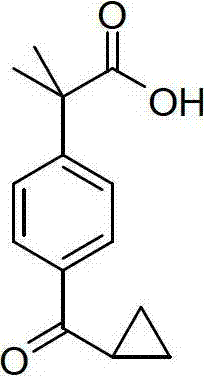
Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid
InactiveCN101585763AReduce pollutionSimple processPreparation from carboxylic acid amidesAlcohol2-methylpropanoic acid
The invention discloses a method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping the alcohol solvent of N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide in the alcohol solvent, agitating and reacting for 10-30h at 20-50 DEG C, extracting, drying and filtering to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in the alcohol solvent of the alkali metal hydroxide, refluxing for 20-40h, and adjusting pH to be 3 by hydrochloric acid, extracting, drying and filtering to obtain 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid, and adding the 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid in an inorganic acid, reacting for 20-30h at 60-100 DEG C, extracting, drying, filtering and crystallizing by ethanol to obtain the product target. The invention has a simple technology, high yield and little pollution.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester
ActiveCN105753692BReduce manufacturing costGentle preparation processPreparation by hydrogen halide split-offOrganic compound preparationSodium bicarbonateIce water
The invention provides a method for synthesizing 2-methyl propionate-[(2S)-4-(2,4-diflurophenyl)-2-hydroxymethyl-4-amylene-1-group] ester.The method includes the steps that 1,2,3-trichloropropane is added dropwise to 1,3-difluorobenzene, and aluminum trichloride is added for a reaction; the mixture is added to a hydrochloric acid solution for extraction, and washing is conducted with a saturated NaHCO3 solution, water and saturated salt solution in sequence; anhydrous Na2SO4 is used for drying and filtering, evaporation is conducted for solvent removal, and 1,3-dichloro-2-(2,4-diflurophenyl) propane is obtained; 1,3-dichloro-2-(2,4-diflurophenyl) propane and potassium hydroxide are added to tert butyl alcohol, and reflux is conducted for 3.5-6 h; tert butyl alcohol is removed, ice water is added, the mixture is neutralized with hydrochloric acid at the temperature of 5 to -5 DEG C to be neutral, dichloromethane is used for three times of extraction, anhydrous Na2SO4 is used for drying and filtering, a distillation product is dissolved in DMSO, a product obtained after diethyl malonate and diethyl malonate react are added and a product obtained after lithium chloride and sodium borohydride react are added and dissolved in methylbenzene, and sodium bicarbonate, Novo SP 435 esterifying enzymes and isobutyric anhydride are added for a reaction; the target product is obtained after washing, crystallization and drying are conducted.The synthesis path is shown in the description.
Owner:山东汉申化工科技有限公司
The synthetic method of ciprofibrate
ActiveCN105152925BReduce pollutionMild conditionsOrganic compound preparationCarboxylic acid esters preparationAcetic acidAlkyl transfer
Owner:HANGZHOU LUPU BIOTECH CO LTD
Method for synthesizing 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid
InactiveCN101585762BFew reaction stepsSimple preparation processPreparation from carboxylic acid amidesAlcoholMethylene Dichloride
The invention discloses a method for synthesizing 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping the alcohol solvent of N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide in the alcohol solvent, agitating and reacting for 10-30h at 20-50 DEG C, extracting, drying and filtering to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in the alcohol solvent of the alkali metal hydroxide, refluxing and reacting for 20-40h, and adjusting the pH of the reaction mixture to be 3 by hydrochloric acid, extracting, drying, filtering and removing methylene dichloride to obtain 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid. The synthetic method of the invention has the advantages of a few reaction steps, simple preparation technology, high yield, little pollution and lowproduction cost.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid
InactiveCN101585764AReduce pollutionFew reaction stepsPreparation from carboxylic acid amidesOrganic acidAlcohol
The invention discloses a method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid, comprising the steps of adding an alkali metal hydroxide in an alcohol solvent, dripping the alcohol solvent of N-methyl-N-methoxyl-2-[4-(4-chlorobutyryl)phenyl]-2-methacrylamide in the alcohol solvent, reacting for 10-30h at 20-50 DEG C, evaporating the solvent to dryness, extracting, drying and filtering to obtain N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide; adding the N-methyl-N-methoxyl-2-(4-cyclopropoxycarbonylphenyl)-2-methacrylamide in an organic acid in a ratio of 1: 2-8, reacting for 20-30h at 60-100 DEG C, extracting, drying, filtering and recrystallizing to obtain the product target. The invention has the advantages of a simple preparation technology, high yield and little pollution.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropionic acid
InactiveCN101585764BReduce pollutionFew reaction stepsPreparation from carboxylic acid amidesAlcohol2-methylpropanoic acid
The invention discloses a method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropionic acid: adding alkali metal hydroxide to an alcohol solvent, and then adding N- Alcohol solution of methyl-N-methoxy-2-[4-(4-chlorobutyryl)phenyl]-2-methylpropionamide, react at 20~50°C for 10~30h, evaporate the solvent to dryness, Extract, dry, and filter to obtain N-methyl-N-methoxy-2-(4-cyclopropoxycarbonylphenyl)-2-methylpropionamide; then N-methyl-N-methoxy -2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanamide is added to the mineral acid, the weight ratio of the two is 1:2~8, react at 60°C~100°C for 20~30h, extract, Dry, filter, and recrystallize to obtain the target product. The invention has the advantages of simple preparation process, high yield and little pollution.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate](https://images-eureka.patsnap.com/patent_img/d7599c35-5536-4f6b-b0d0-fc7158e1c079/A20091009941700051.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate](https://images-eureka.patsnap.com/patent_img/d7599c35-5536-4f6b-b0d0-fc7158e1c079/A20091009941700052.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate](https://images-eureka.patsnap.com/patent_img/2447d491-d551-45a1-903b-2787ccc646f4/GSB00000618069700021.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate](https://images-eureka.patsnap.com/patent_img/2447d491-d551-45a1-903b-2787ccc646f4/GSB00000618069700022.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate](https://images-eureka.patsnap.com/patent_img/30194c15-a91f-4f8a-b11c-096f3c112630/A20091009941500051.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methacrylate](https://images-eureka.patsnap.com/patent_img/30194c15-a91f-4f8a-b11c-096f3c112630/A20091009941500052.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid](https://images-eureka.patsnap.com/patent_img/6390c62e-5e5a-433d-af4c-586cd8896840/A20091009941800041.PNG)
![A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester](https://images-eureka.patsnap.com/patent_img/6b42f10b-3f3e-4101-80af-627de710ab3a/BDA0000953377450000011.png)
![A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester](https://images-eureka.patsnap.com/patent_img/6b42f10b-3f3e-4101-80af-627de710ab3a/BDA0000953377450000012.png)
![A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester A method of synthesizing 2-methylpropionic acid-[(2s)-4-(2,4-difluorophenyl)-2-hydroxymethyl-4-penten-1-yl] ester](https://images-eureka.patsnap.com/patent_img/6b42f10b-3f3e-4101-80af-627de710ab3a/BDA0000953377450000021.png)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropanoic acid](https://images-eureka.patsnap.com/patent_img/7e7c5b22-03b5-4852-bf1d-0f0df65c3c3d/A20091009942000041.PNG)
![Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropionic acid Method for synthesizing 2-[4-(4-chlorobutyryl)phenyl]-2-methylpropionic acid](https://images-eureka.patsnap.com/patent_img/9bbe6c21-d953-43b7-8e8a-24b25af6e0b2/GSB00000618008700011.PNG)