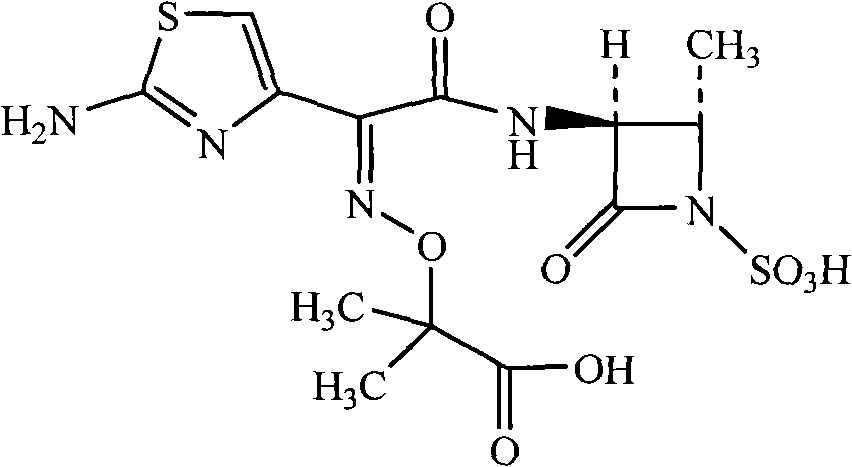
Method for synthesizing aztreonam compound
A synthetic method and compound technology, applied in the field of medicine, can solve the problems of not being suitable for large-scale industrial production, not conducive to environmental protection, etc., and achieve the effects of saving production costs, reducing reaction time, and speeding up the reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 175g of purified water and 140g of methanol to the reaction vessel, add 20g of trans-3(S)-amino-4-methyl-2-oxo-1-azetidinanesulfonic acid, stir and cool down to -20°C . Keeping the temperature, start to add triethylamine dropwise until the pH value of the solution is 6.0. Begin to add (Z)-2-[(2-aminothiazol-4-yl)-(benzothiazol-2-ylthiocarbonyl)methyleneaminooxy]-2-methylpropionic acid tert-butyl ester 60g , During the feeding process, triethylamine is added dropwise, the pH value is always controlled between 5.0 and 9.0, the temperature is between -20 and -10, and the time is controlled within 2 hours. Continue to react for 2 hours. Filter, lower the temperature to -10°C, adjust the pH value to 1.5 with concentrated hydrochloric acid, white crystals are precipitated, keep warm and crystallize for 2 hours, filter to obtain tert-butylaztreonam.
[0031]Add tert-butylaztreonam into 3 times the amount of acetic acid and water mixed solvent (acetic acid: water = 5:5, ...
Embodiment 2
[0033] Add 200g of purified water, 100g of ethanol, and 100g of acetone into the reaction vessel, add 28g of trans-3(S)-amino-4-methyl-2-oxo-1-azetidinanesulfonic acid, stir and cool down to -30°C. Keeping the temperature, start to add triethylamine dropwise until the pH value of the solution is 8.0. Add (Z)-2-[(2-aminothiazol-4-yl)-(benzothiazol-2-ylthiocarbonyl)methyleneaminooxy]-2-methylpropionic acid tert-butyl ester 84g, During the feeding process, the pH value is always controlled between 5.0 and 9.0, and the temperature is between -20 and 0°C. The control is added within two hours. Continue to react for 2 hours, add activated carbon and stir for 0.5 hours. Filter, lower the temperature to -10°C, adjust the pH value to 1.0-1.5 with concentrated hydrochloric acid, white crystals precipitate, keep warm for 2 hours, and filter to obtain tert-butylaztreonam.
[0034] Add tert-butylaztreonam into a mixed solvent of 3 times the amount of acetic acid and water (acetic acid:...
Embodiment 3
[0036] Add 350kg of purified water, 280kg of acetone, and 40kg of trans-3(S)-amino-4-methyl-2-oxo-1-azetidinanesulfonic acid into the reaction vessel, stir and cool down to -20°C . Keeping the temperature, start to add triethylamine dropwise until the pH value of the solution is 5.0. Add (Z)-2-[(2-aminothiazol-4-yl)-(benzothiazol-2-ylthiocarbonyl)methyleneaminooxy]-2-methylpropionic acid tert-butyl ester 120kg, During the feeding process, the pH value is always controlled between 5.0 and 9.0, and the temperature is between -30 and -20°C. The control is added within 6 hours. Continue to react for 2 hours, add activated carbon and stir for 0.5 hours. Filter, lower the temperature to -20°C, adjust the pH value to 1.0-1.5 with concentrated hydrochloric acid, white crystals are precipitated, keep warm and crystallize for 2 hours, filter to obtain tert-butylaztreonam.
[0037] Add tert-butylaztreonam into 3 times the amount of acetic acid and water mixed solvent (acetic acid: wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com