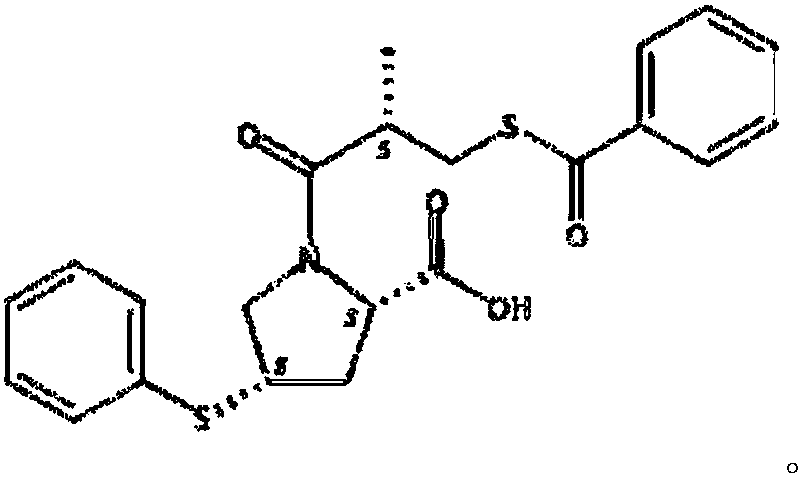
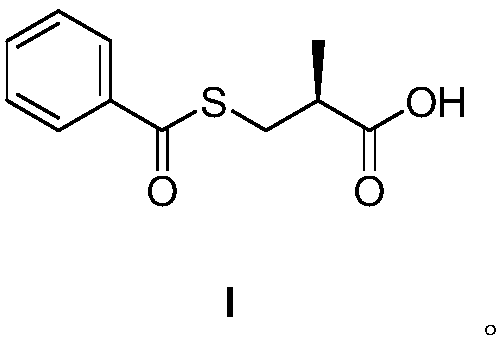
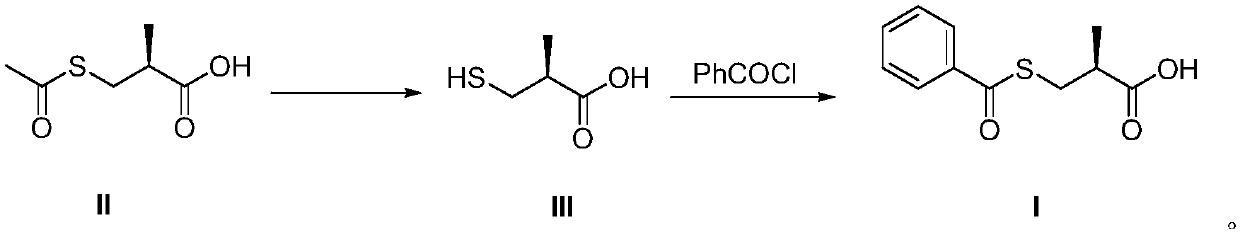
Method for preparing S-(-)-Benzoylthio-2-methylpropanoic acid compound
A technology of benzoyl mercapto and methylpropionic acid, which is applied in the field of synthesis of zofenopril intermediates, can solve the problems of high price of thiobenzoic acid, large environmental pollution, and low total yield, and achieve high yield High, cheap raw materials, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Under the protection of nitrogen, add 21.5g of sodium hydroxide, 90mL of drinking water and 0.4g of zinc powder to the three-necked flask successively, cool down to 0°C, and slowly add 20g of S-(-)-3-acetylmercapto-2-methylpropionic acid dropwise , insulation reaction 3h. After the reaction, the pH was adjusted to 9.0 with concentrated hydrochloric acid. Control the feed liquid temperature to be 15°C, add dropwise 20g of benzoyl chloride and 36mL of 4mol / L sodium hydroxide aqueous solution. After the reaction, use concentrated hydrochloric acid to adjust the pH to 2.0, add 80 mL of ethyl acetate for extraction, and add 120 mL of n-heptane to the ethyl acetate layer, stir and crystallize for 4 hours, filter, and dry to obtain 19.4 g of S-(- )-benzoylmercapto-2-methylpropionic acid, yield: 71.9%, HPLC: purity: 99.90%, optical purity: 99.2%.
example 2
[0023] Under the protection of nitrogen, add 21.5g of sodium hydroxide, 90mL of drinking water and 0.2g of aluminum powder to the three-necked flask successively, cool down to 0°C, and slowly add 20g of S-(-)-3-acetylmercapto-2-methylpropionic acid dropwise , insulation reaction 3h. After the reaction, the pH was adjusted to 9.0 with concentrated hydrochloric acid. Control the feed liquid temperature to 15°C, add dropwise 22g p-toluoyl chloride and 36mL 4mol / L sodium hydroxide aqueous solution. After the reaction was finished, use concentrated hydrochloric acid to adjust the pH to 2.0, add 80 mL of ethyl acetate for extraction, and after layering, add 120 mL of n-heptane to the ethyl acetate layer, stir and crystallize for 4 hours, filter, and dry to obtain 19.2 g of S-(- )-benzoylmercapto-2-methylpropionic acid, yield: 66.7%, HPLC: purity: 99.0%, optical purity: 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com