Method for synthesizing alecensa hydrochloride intermediate 2-(4-ethyl-3-iodophenyl)-2-methylpropanoic acid
A technology of methacrylic acid and ethyl phenyl, which is applied in the field of preparation of organic compounds, can solve the problems of environmental protection pressure, production equipment corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
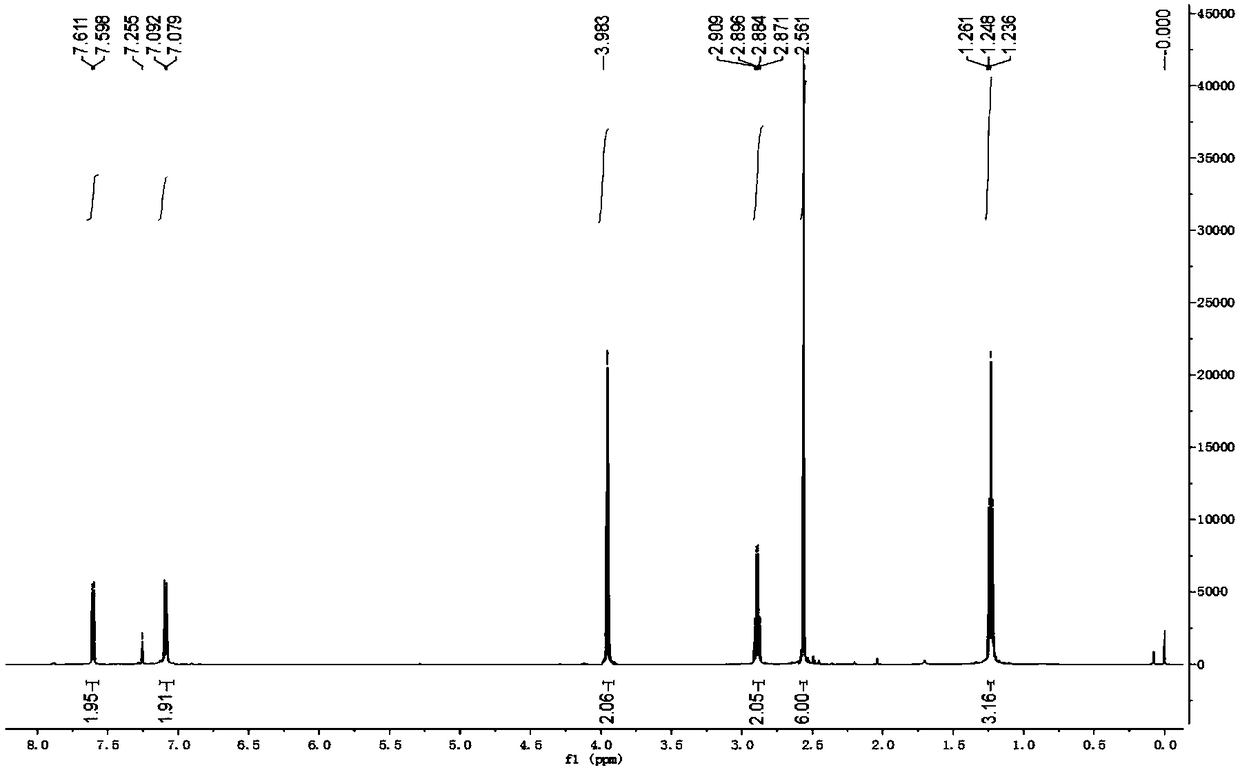
[0055] The synthetic method of 2-(4-ethyl-3-iodophenyl)-2-methylpropionic acid, the steps are as follows:
[0056] a. Preparation of 1-chloro-2-(4-ethylphenyl)-2-methylpropane (compound 1a):
[0057] At normal temperature, in reactor A with condensing reflux device, add ethylbenzene (159.2g, i.e. 1.5mol), H 2 SO 4 (80%H 2 SO 4 6.13g, or 0.05mol H 2 SO 4 ), stirred evenly, and added 3-chloro-2-methyl-1-propene (90.6g, namely 1.0mol) dropwise with stirring at 40°C. Water, after stirring, let it stand for stratification, remove the lower aqueous phase containing sulfuric acid, leave the organic phase, add an appropriate amount of water and an appropriate amount of saturated sodium chloride aqueous solution to the organic phase again, stir and then let it stand for stratification , discard the lower aqueous phase and saturated aqueous sodium chloride solution; then purify the organic phase by distillation and collect fractions to obtain 1-chloro-2-(4-ethylphenyl)-2-methylpr...
Embodiment 2
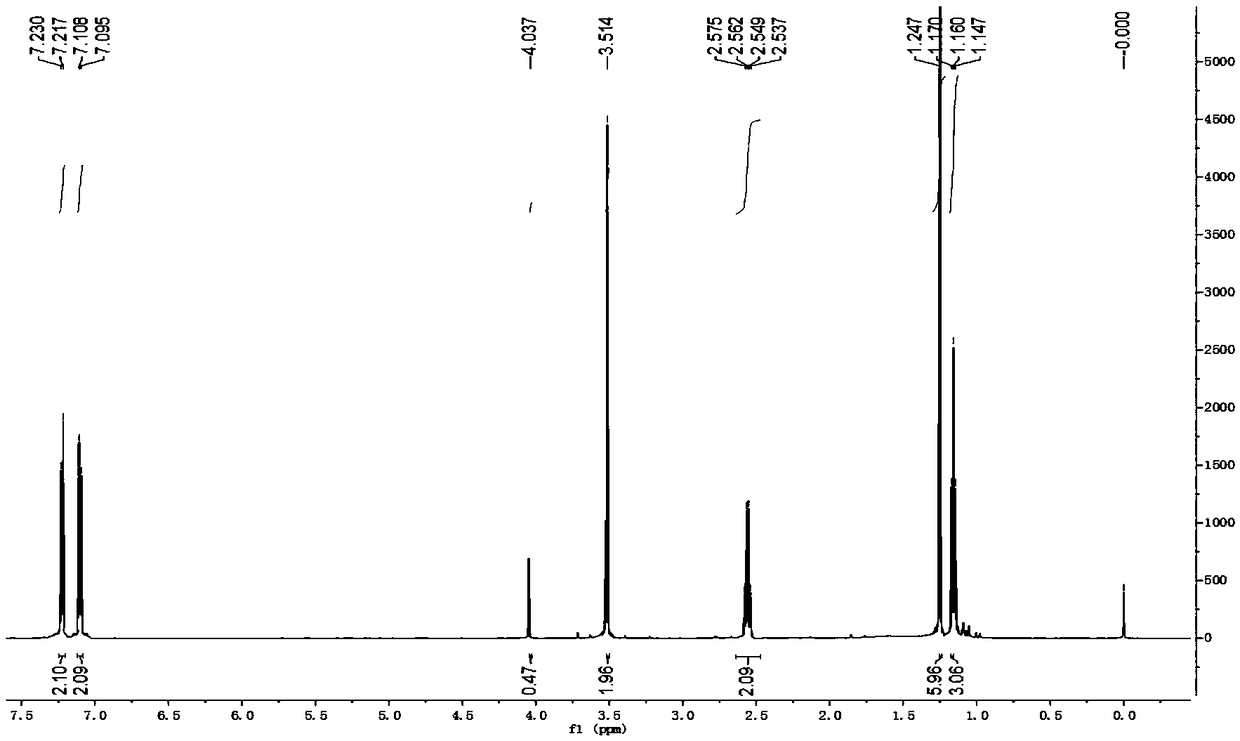
[0066] The synthetic method of 2-(4-ethyl-3-iodophenyl)-2-methylpropionic acid, the steps are as follows:
[0067] a. Preparation of 1-chloro-2-(4-ethylphenyl)-2-methylpropane (compound 1a):
[0068] At normal temperature, in reactor A with condensing reflux device, add ethylbenzene (169.9g, i.e. 1.6mol), phosphoric acid (85% phosphoric acid 11.5g, i.e. 0.10mol H 3 PO 4 ), stirred evenly, and added 3-chloro-2-methyl-1-propene (90.6g, namely 1.0mol) dropwise with stirring at 17°C. Water, after stirring, let it stand for stratification, remove the lower aqueous phase containing phosphoric acid, leave the organic phase, add an appropriate amount of water and an appropriate amount of saturated sodium chloride aqueous solution to the organic phase again, stir and let it stand for stratification , discard the lower aqueous phase and saturated aqueous sodium chloride solution; then purify the organic phase by distillation and collect fractions to obtain 1-chloro-2-(4-ethylphenyl)-2...
Embodiment 3
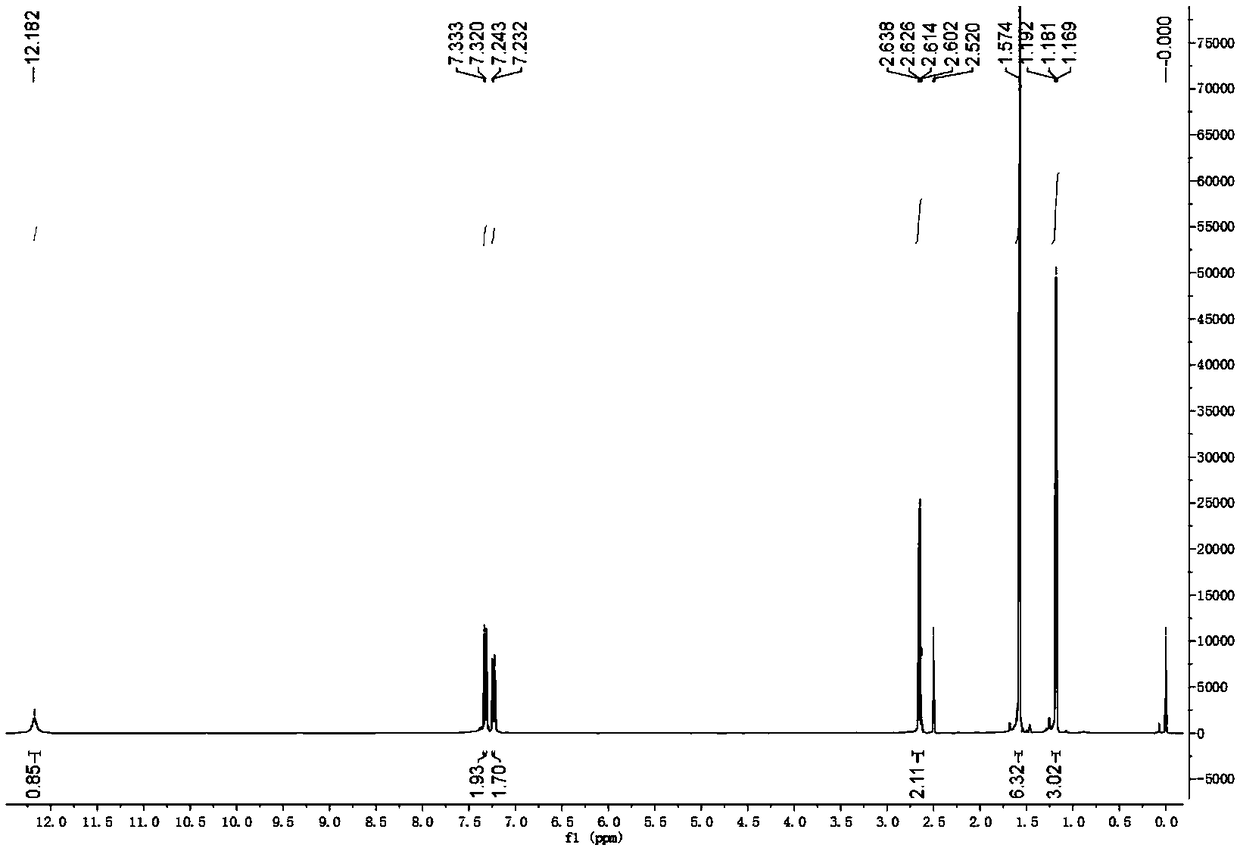
[0077] The synthetic method of 2-(4-ethyl-3-iodophenyl)-2-methylpropionic acid, the steps are as follows:
[0078] a. Preparation of 1-chloro-2-(4-ethylphenyl)-2-methylpropane (compound 1a):
[0079]At room temperature, add ethylbenzene (180.5g, or 1.7mol) and p-toluenesulfonic acid (24.1g, or 0.14mol) to reactor A with a condensing reflux device, stir evenly, and add dropwise at 24°C 3-Chloro-2-methyl-1-propene (90.6g, ie 1.0mol), after the dropwise addition, after the reaction at 36°C for 8 hours, add an appropriate amount of water, stir and let stand, carry out layering, remove The lower part contains the water phase of p-toluenesulfonic acid, leaving the organic phase, adding an appropriate amount of water and an appropriate amount of saturated sodium chloride aqueous solution to the organic phase again, stirring and standing for layering, discarding the lower aqueous phase, saturated Aqueous sodium chloride solution; the organic phase was distilled and purified, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com