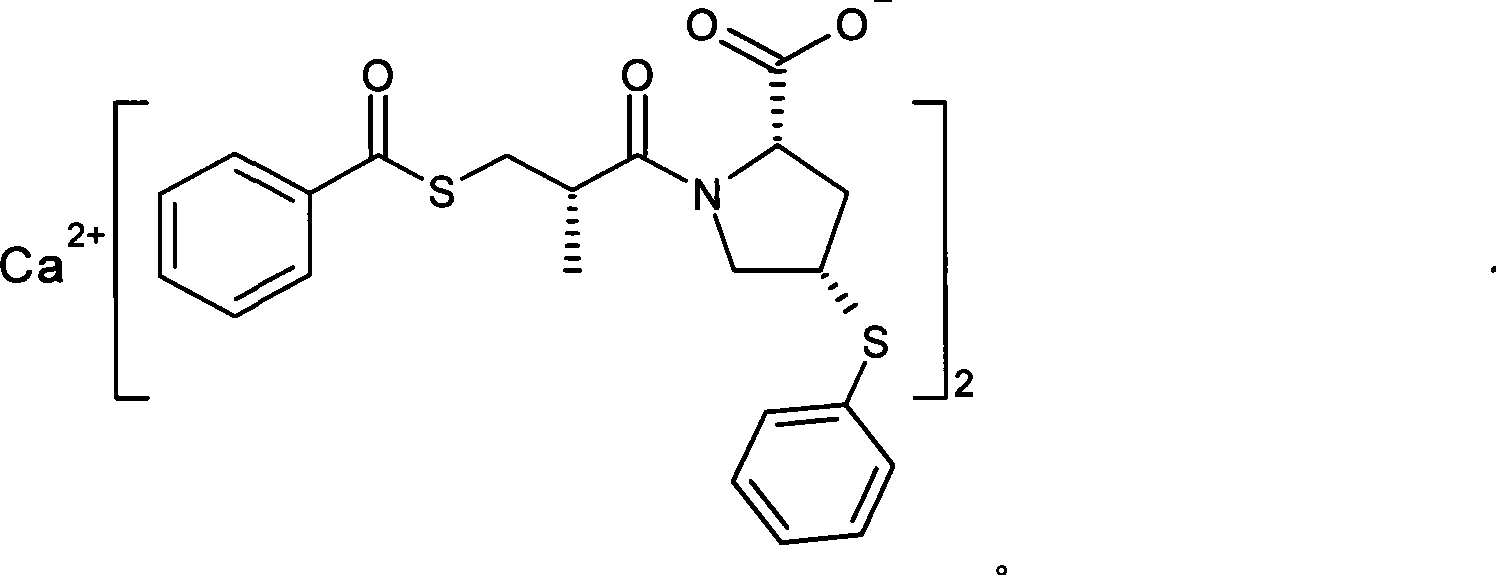
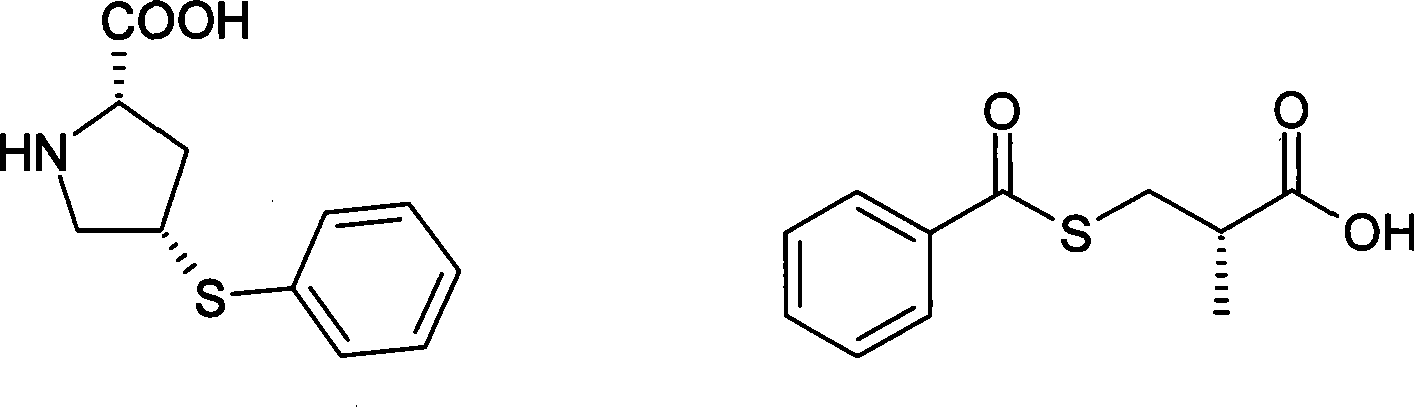
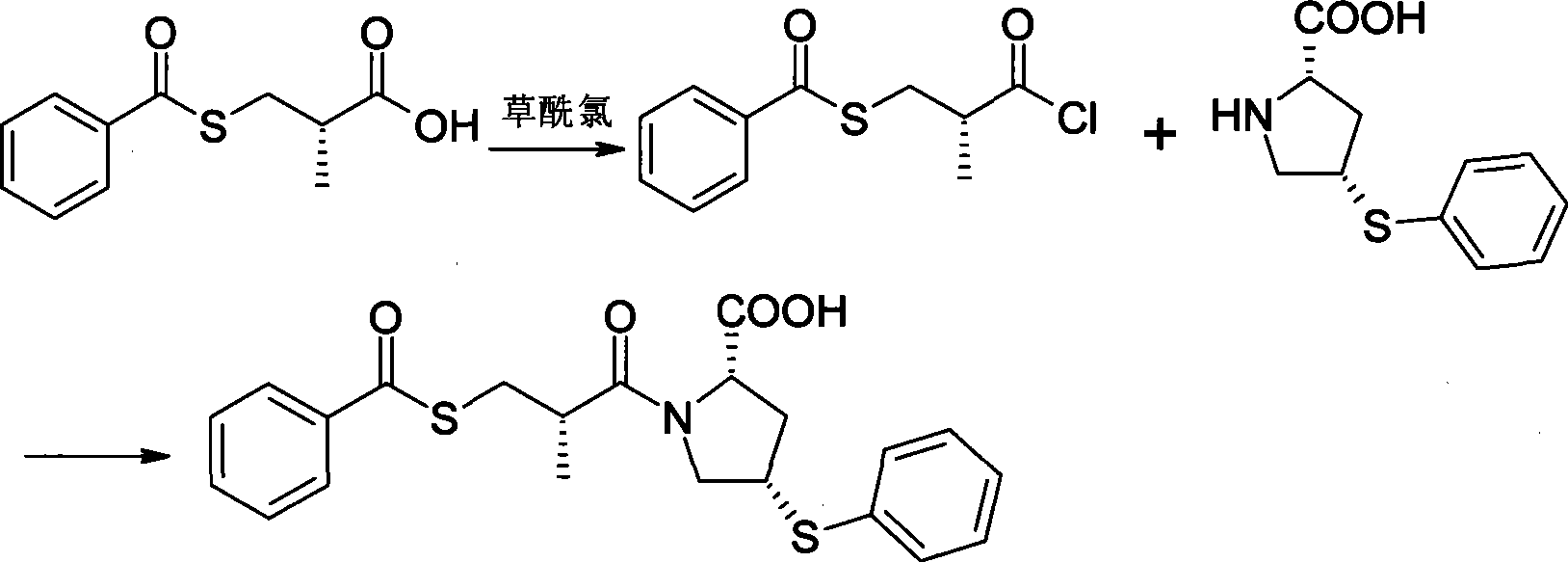
Method for synthesizing Zofenopril
A technology of zofenopril calcium and a synthesis method, which is applied in the field of drug synthesis and can solve problems such as difficulty in refining, insoluble calcium salts and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0095] Example 1 (synthesis of N-acetyl-L-hydroxyproline methyl ester):
[0096] Raw materials and proportioning (see Table 1)
[0097] Table 1
[0098]
[0099]
[0100] operate:
[0101] Add 600g of N-acetyl-hydroxyproline and 1000ml of anhydrous methanol into a 3000ml three-neck flask, stir for 45min to dissolve, then add 120g of p-toluenesulfonic acid, stir at room temperature for 48h, TLC (see below for the method) to detect the end point, raw materials The point basically disappears as the end point. Add solid sodium bicarbonate to adjust pH=7, filter, add anhydrous Na to the filtrate 2 SO 4 Dry, filter, concentrate under reduced pressure at 50°C to remove the solvent, add the oily residue to acetone, stir and mix, and a white precipitate precipitates out, filter, wash the solid with acetone, and concentrate the filtrate to obtain 605 g of a light yellow oil (Intermediate 1). 93.2%
example 2
[0102] Synthesis of Example 2 (N-acetyl-trans-4-tosyl-L-hydroxyproline methyl ester)
[0103] Raw materials and proportioning (see Table 2)
[0104] Table 2
[0105]
[0106] operate:
[0107] Put 602g of intermediate (1) and 1200ml of pyridine in a 3000ml dry three-neck flask, stir in an ice bath for 30min, add 735g of p-toluenesulfonyl chloride in batches at (0-10°C), stir to form a solid, and store in the refrigerator Stand overnight, add 600ml of water to dissolve the solid, extract with 800ml of dichloromethane x 3 times, combine the organic layers, and wash with dichloromethane, water, 2mol / l HCL, saturated sodium bicarbonate, and water twice each. The organic layer was dried and filtered, and the solvent was evaporated to obtain 928 g of a reddish-brown oil (Intermediate 2), with a yield of 84.5%.
example 3
[0108] Synthesis of Example 3 (N-acetyl-cis-4-phenylthio-L-proline methyl ester)
[0109] Raw materials and proportioning (see Table 3)
[0110] table 3
[0111]
[0112]
[0113] operate:
[0114] Add 3000ml of absolute ethanol into a dry 5000ml three-neck flask, stir, and slowly add 103g of chopped metal sodium in batches under ice-bath cooling, add 450g of thiophenol after dissolving, stir for 1h, and cool down in a water bath to At room temperature, add 926 g of intermediate (2) at one time, and stir overnight at room temperature (the temperature should not be too high). Ethanol was evaporated under reduced pressure, 1000ml of water and 1000mL of dichloromethane were added to the residue to dissolve the solid, the organic layer was separated, and the aqueous phase was extracted with 1000ml of dichloromethane, the organic layers were combined, washed twice with water, and anhydrous Na 2 SO 4 After drying, filtering, and distilling off th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com