Process for preparing sulindac
A technology of sulindac and indene acetic acid is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, which can solve the problems of increased COD in wastewater, incomplete reaction, and generation of impurities, and achieves reduction in production cost and reaction time. Short, small amount of impurities produced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
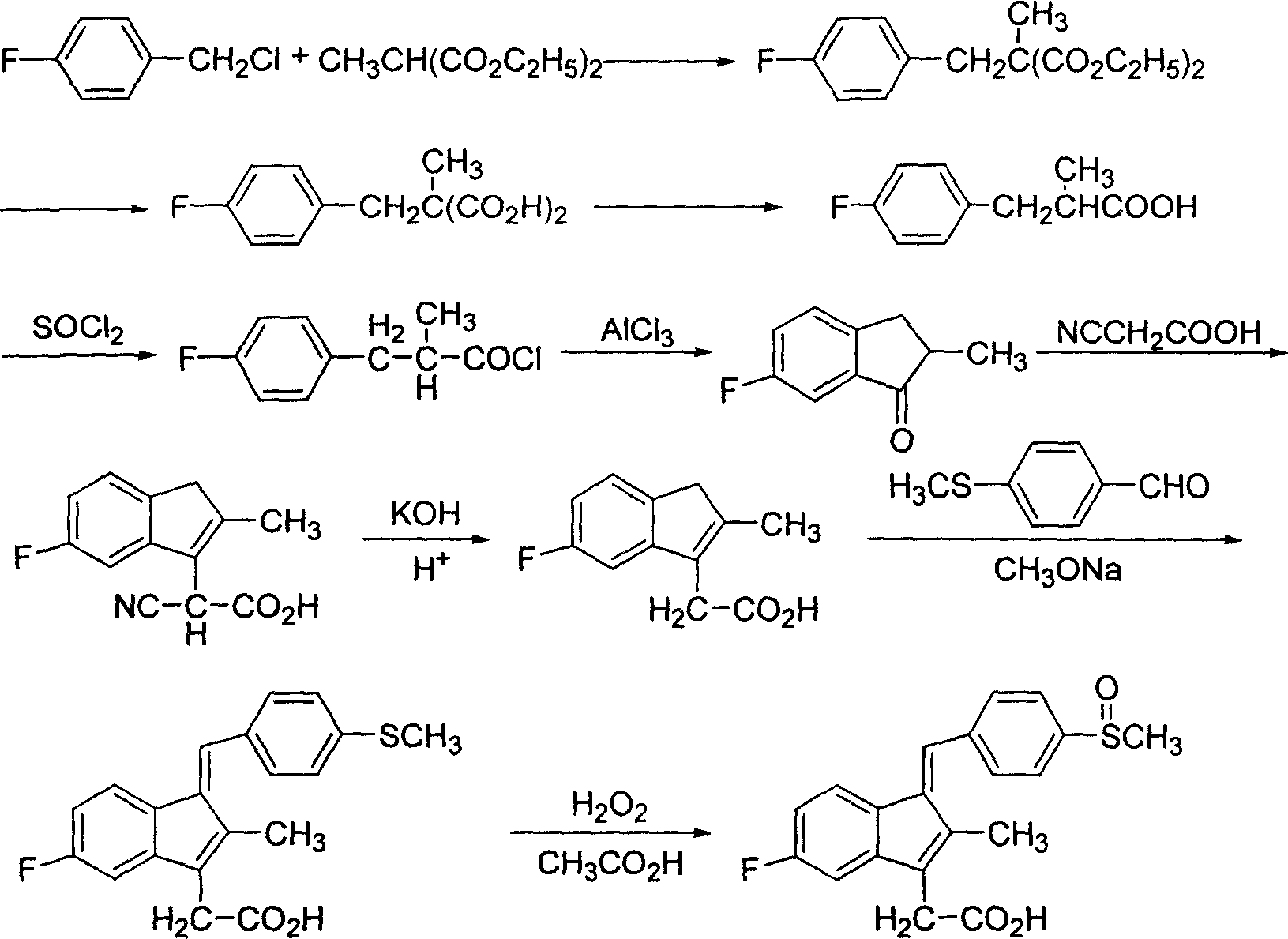
[0025] Preparation of 2-(4-fluorophenyl)-2-methylmalonic acid
[0026] Add 100g of industrial sodium methoxide solution into a 1000ml reaction bottle, then add 100g of anhydrous methanol, heat to reflux, slowly add 72.7g (0.417mol) diethyl methylmalonate dropwise, after the dropwise addition is complete, reflux for 45min, Add 59.8g (0.414mol) of p-fluorobenzyl chloride dropwise, and reflux for about 4.5 hours after dropping. The end point of the reaction is detected by TLC. After the reaction is completed, anhydrous methanol is recovered, and 220 g of 25% KOH solution is added to the above-mentioned reactor, and the reaction is refluxed for about 9 hours. Adjust the pH to 1 with concentrated hydrochloric acid, extract three times with dichloromethane, and concentrate to obtain 75.2 g of off-white solid, mp.138.5-139.5°C, two-step molar yield 80.6%.
[0027] Preparation of 3-(4-fluorophenyl)-2-methylpropionic acid
[0028] Put 75.2g of the off-white solid obtained in the previ...
Embodiment 2
[0041] Preparation of 2-(4-fluorophenyl)-2-methylmalonic acid
[0042] Industrial sodium ethoxide was used instead of sodium methoxide, 100 g of absolute ethanol was used instead of absolute methanol as the solvent, and the rest was the same as in Example 1 to obtain 75.3 g of an off-white solid with a two-step molar yield of 80.7%.
[0043] Preparation of 3-(4-fluorophenyl)-2-methylpropionic acid
[0044] Put 75.3 g of the off-white solid obtained in the previous step into a 250 ml four-necked bottle equipped with a reflux tube and a drying tube, stir and heat up to 145° C., react for 1.5 h, and the rest are the same as in Example 1.
[0045] Preparation of 6-fluoro-2-methylindanone
[0046] Replace 54.6g of anhydrous aluminum trichloride with 55.8g of anhydrous zinc chloride, all the other are the same as in embodiment 1, obtain off-white solid 40.2g, mp.34~34.5 ℃.
[0047] Preparation of 5-fluoro-2-methyl-3-indeneacetic acid
[0048] Example 1 After refluxing for 12 hour...
Embodiment 3
[0053] Preparation of 2-(4-fluorophenyl)-2-methylmalonic acid
[0054] Sodium tert-butoxide was used instead of sodium methoxide, 100 g of tert-butanol was used instead of anhydrous methanol as the solvent, and the rest were the same as in Example 1 to obtain 75.5 g of off-white solid with a two-step molar yield of 80.8%.
[0055] Preparation of 3-(4-fluorophenyl)-2-methylpropionic acid
[0056] Put 75.5 g of the off-white solid obtained in the previous step into a 250 ml four-necked bottle equipped with a reflux tube and a drying tube, stir and raise the temperature to 155° C., react for 1.0 h, and the rest are the same as in Example 1.
[0057] Preparation of 6-fluoro-2-methylindanone
[0058] Carbon disulfide was used instead of dichloromethane as the cyclization solvent, and the rest was the same as in Example 1 to obtain 40.8 g of off-white solid.
[0059] Preparation of 5-fluoro-2-methyl-3-indeneacetic acid
[0060] Embodiment 1 adds cyanoacetic acid and ammonium acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com