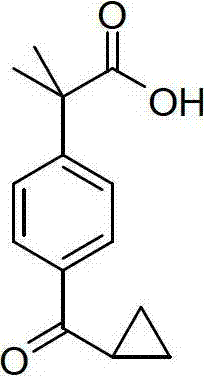
Method for synthesizing 2-(4-cyclopropoxycarbonylphenyl)-2-methylpropanoic acid
A technology of cyclopropylcarbonylphenyl and methylpropionic acid, which is applied in the field of synthesizing 2-(4-cyclopropylcarbonylphenyl)-2-methylpropionic acid, can solve the problem of many steps, high cost and high production cost. The efficiency is not very high, and the effect of simple preparation process, low production cost and few reaction steps is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 16.00 g (0.40 mol) of sodium hydroxide and 150 ml of anhydrous methanol to a 250 ml three-necked flask and stir evenly, then, while stirring, 15.50 g (0.05 mol) of N-methyl-N-methoxy-2- [4-(4-Chlorobutanoyl)phenyl]-2-methylpropanamide is dissolved in 50 ml of anhydrous methanol to form a uniformly stirred solution, which is added dropwise to a three-necked flask, and stirred at 30°C for reaction after the dropwise addition 18 hours. After the reaction was completed, anhydrous methanol was distilled off under reduced pressure, 100 ml of water and 100 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. The extracts containing the dichloromethane layer were combined, dried with anhydrous sodium sulfate, filtered, and the dichloromethane was distilled off under reduced pressure to obtain the product N-methyl-N-methoxy-2-(4-cyclopropylcarbonylbenzene Base)-2-me...
Embodiment 2
[0029] Add 20.50 g (82%, 0.30 mol) of potassium hydroxide and 150 ml of anhydrous methanol into a 250 ml three-necked flask and stir evenly, then, while stirring, add 15.50 g (0.05 mol) of N-methyl-N-methoxy -2-[4-(4-Chlorobutanoyl)phenyl]-2-methylpropanamide was dissolved in 50 ml of anhydrous methanol to form a uniformly stirred solution that was added dropwise to the three-necked flask. The reaction was stirred at °C for 30 hours. After the reaction was completed, anhydrous methanol was distilled off under reduced pressure, 100 ml of water and 100 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. The extracts containing the dichloromethane layer were combined, dried with anhydrous sodium sulfate, filtered, and the dichloromethane was distilled off under reduced pressure to obtain the product N-methyl-N-methoxy-2-(4-cyclopropylcarbonylbenzene base)-2-methylpropan...
Embodiment 3
[0032] Add 20.00 g (0.50 mol) of sodium hydroxide and 150 ml of absolute ethanol to a 250 ml three-necked flask, stir well, then, while stirring, add 15.50 g (0.05 mol) of N-methyl-N-methoxy-2 -[4-(4-Chlorobutyryl)phenyl]-2-methylpropanamide dissolved in 50 ml of absolute ethanol to form a uniformly stirred solution was added dropwise to the three-necked flask. The reaction was stirred for 30 hours. After the reaction was completed, absolute ethanol was distilled off under reduced pressure, 100 ml of water and 100 ml of dichloromethane were added to the residue, the layers were separated, and the aqueous layer was extracted twice with 100 ml of dichloromethane respectively. Combine the extracts containing the dichloromethane layer, dry over anhydrous sodium sulfate, filter, distill off the dichloromethane under reduced pressure to obtain the product N-methyl-N-methoxy-2-(4-cyclopropylcarbonylphenyl) - 12.65 g (0.046 mol) of 2-methylpropanamide, melting range 82-83°C, yield 92...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com