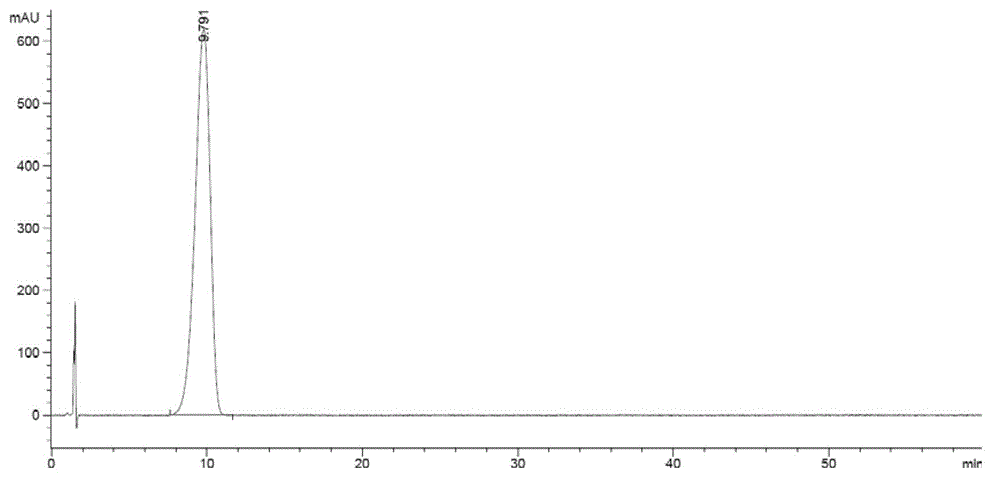
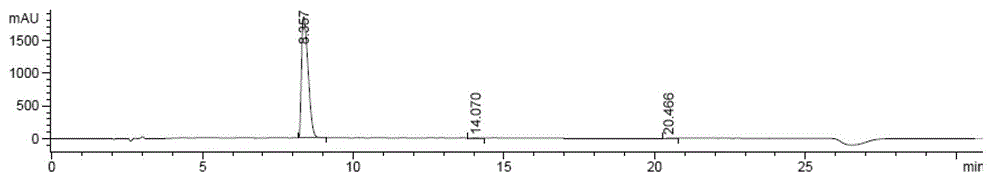
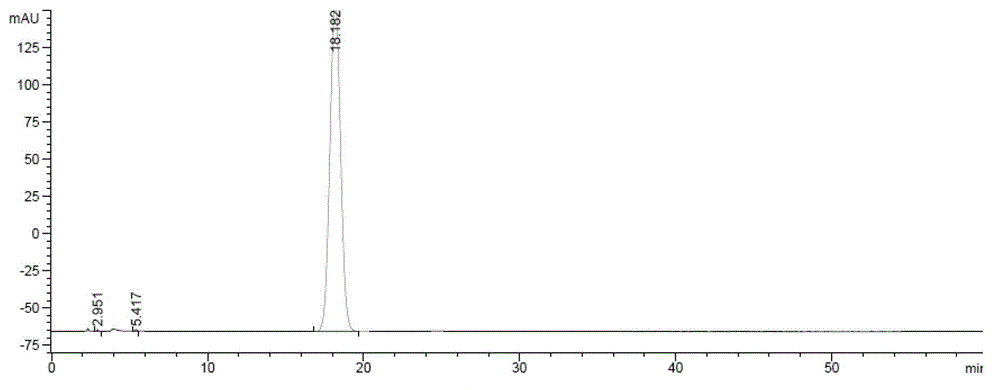
Method for preparing zofenopril calcium
A zofenopril calcium and compound technology, applied in the field of medicinal chemistry, can solve the problems of difficult dissolution and refinement of calcium salts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0056] Preparation of Example 1 N-acetyl-L-hydroxyproline methyl ester (compound 1):
[0057] Raw materials and proportioning (see Table 1)
[0058] Table 1
[0059]
[0060] operate:
[0061] Add 1732g of N-acetyl-L-hydroxyproline and 2500ml of anhydrous methanol into a 5000ml reaction kettle, stir for 45min to dissolve, then add 380g of p-toluenesulfonic acid, stir at room temperature for 48h, TLC (using silica gel GF254 thin-layer plate, two Chloromethane / ethyl acetate / methanol=1 / 5 / 0.2 developer, phosphomolybdic acid color) detection end point, the raw material point basically disappears as the end point. Add solid sodium bicarbonate to adjust pH=7~8, filter, add anhydrous Na to the filtrate 2 SO 4 Dry, filter, concentrate under reduced pressure at 40-50°C to remove the solvent, add the oily residue to 4000ml of acetone, stir and mix, a white precipitate precipitates out, filter, wash the solid with acetone, and concentrate the filtrate to obtain 1960g of light yel...
example 2
[0062] Preparation of Example 2 (N-acetyl-trans-4-p-toluenesulfonyl-L-hydroxyproline methyl ester) (compound 2)
[0063] Raw materials and proportioning (see Table 2)
[0064] Table 2
[0065]
[0066] operate:
[0067] Put 1960g of the crude product of compound (1) (calculated as 1872g effective amount) and 2700ml of pyridine in a 5000ml dry reaction kettle, stir for 30min under ice bath, add 2288g of p-toluenesulfonyl chloride in batches at 0-10°C, and add After completion, the temperature was naturally raised to 15-25°C for 12 hours, and solids were precipitated. TLC (using silica gel GF254 thin-layer plate, dichloromethane / ethyl acetate / methanol=1 / 5 / 0.2 developer, iodine color development) detection end point, The raw material point basically disappears as the end point. Add 5600ml of water to dissolve the solid, extract with 3600ml of dichloromethane x 3 times, combine the organic layers, wash with 2mol / l hydrochloric acid 4 times, water once, saturated sodium bic...
example 3
[0068] The preparation of example 3 thiophenol
[0069] Raw materials and proportioning (see Table 3)
[0070] table 3
[0071]
[0072] operate:
[0073] Under strong ventilation conditions, add 3750g of sodium thiophenate aqueous solution (≥40%) into a 20L clean reactor, and add about 2500mL of 20% dilute hydrochloric acid to the system under stirring conditions to adjust to acidic (pH=1 ~2), complete, continue to stir at room temperature for 30min. After standing still, the lower aqueous phase was separated, the organic phase was washed three times with saturated brine, the organic layer was dried over anhydrous sodium sulfate for more than 10 h, and the solid was removed by filtration to obtain 1218 g of a light-colored oily liquid with a yield of 97%. Concentrated treatment of hazardous waste: Combine all water phases, and the washing liquid when cleaning the reactor or other equipment that has been in contact with thiophenol with ethanol or acetone, soak in 40% sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com