Pharmaceutical composition containing micronized fexofenadine hydrochloride
A technology of fexofenadine hydrochloride and its composition, which is applied in the field of micronized fexofenadine hydrochloride pharmaceutical composition and its pharmaceutical preparations, can solve the problems of satisfactory bioavailability and discomfort for clinical use, and achieve the purpose of alleviating itching The effect of the number of wheals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
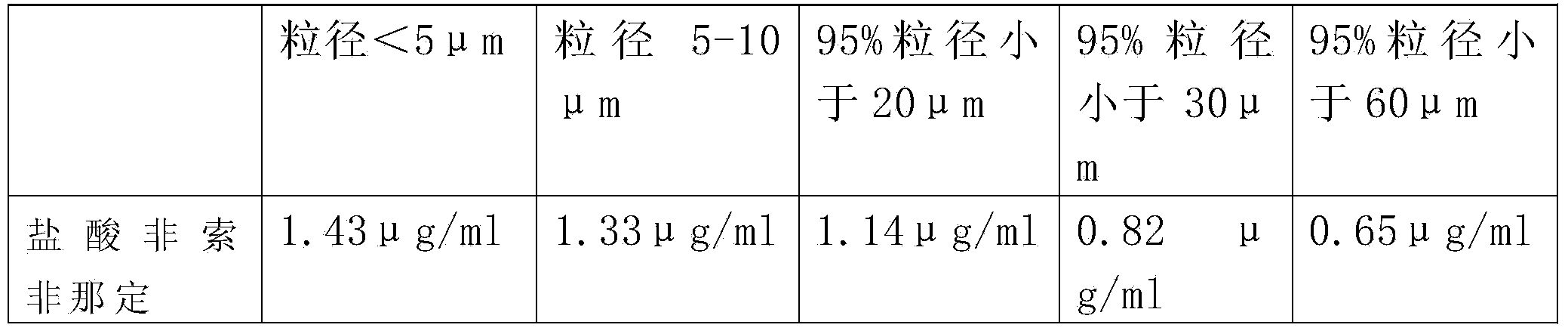
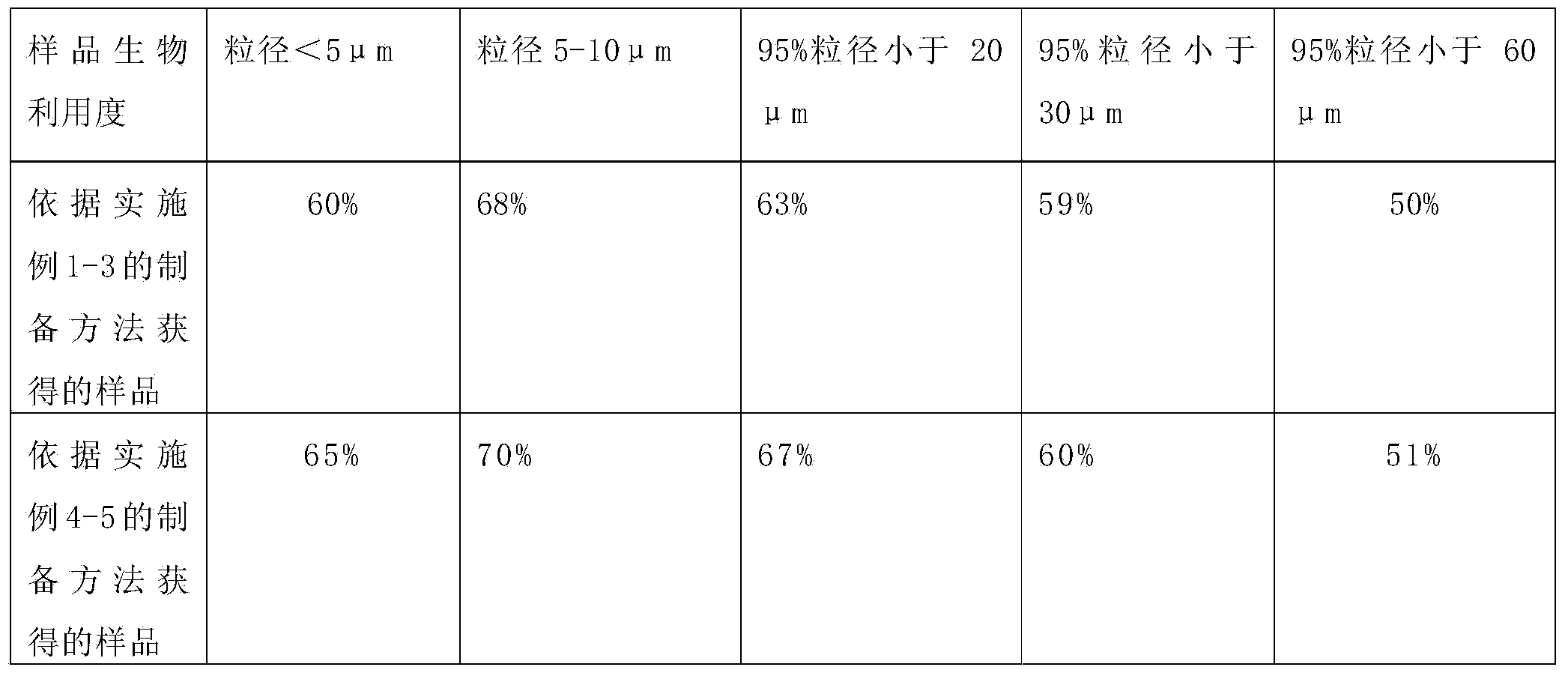
[0043] Fexofenadine hydrochloride raw material is ultrafinely pulverized using a mechanical pulverizer, the pulverized raw material particles are collected, and the particle size of the micropowder is measured using a Malvern laser particle size analyzer. The particle size at 90% of the cumulative volume is below 30 μm, namely: Micronized fexofenadine hydrochloride.
preparation example 2
[0045] Fexofenadine hydrochloride raw material is ultrafinely pulverized by jet mill, the pulverized raw material particles are collected, and the particle size of the fine powder is measured by Malvern laser particle size analyzer, and the particle size at 90% cumulative volume is below 20 μm, namely, Micronized fexofenadine hydrochloride.
preparation example 3
[0047] (1) Fexofenadine hydrochloride is pre-crushed by a fluid energy mill using collision technology to make particles with a particle size of 80 μm,
[0048] (2) Adopt CWJ-30 type superfine pulverizer to carry out superfine pulverization to the above-mentioned coarse particles, pulverize into 5-10 μ m fine powder,
[0049] Grinding conditions: the air temperature after freeze-drying is 6°C, the water content is 0.5%, the pressure when injected into the ultrafine pulverizer is 0.8MPa, the working pressure of the ultrafine pulverizer is 0.8MPa, the internal working temperature is 6°C, and the pulverization time is 50min.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com