Method for preparing myricetin/HP-beta-CD inclusion compound superfine granules through supercritical CO2 anti-solvent technique
An ultra-fine particle, anti-solvent technology, used in anti-toxic agents, anti-inflammatory agents, drug combinations, etc., to achieve green and efficient processes, reduce raw material losses, and overcome the effects of organic solvent residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
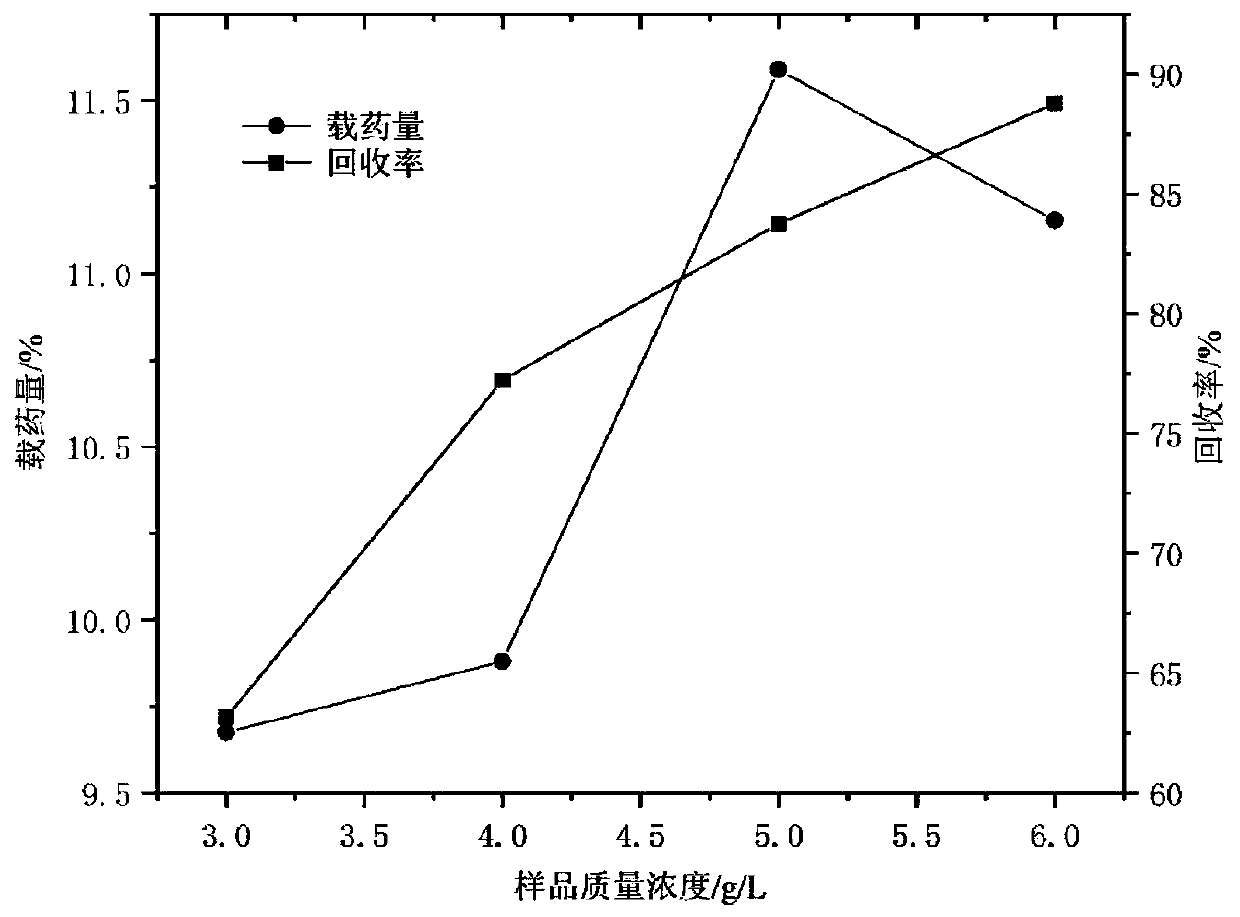
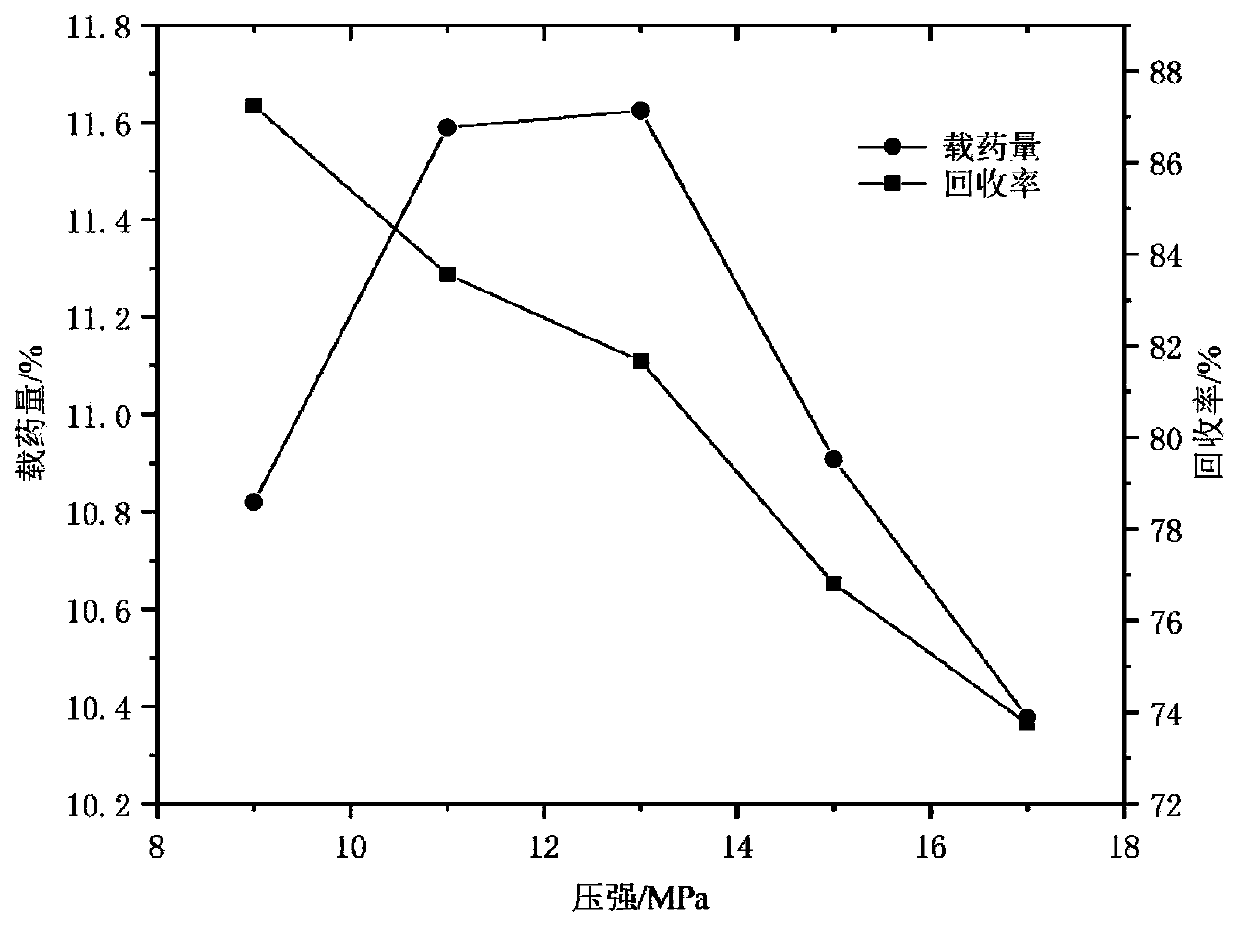
[0040] Example 1: Single factor method to investigate the influence of various factors on the drug loading and recovery rate of myricetin / HP-β-CD inclusion complex ultrafine particles
[0041] Single factor experiment: CO 2 Effect of Flow Rate on the Recovery Rate and Morphology of Myricetin / HP-β-CD Inclusion Complex Ultrafine Particles
[0042] Under the conditions of crystallization pressure of 11MPa, crystallization temperature of 40°C, mass concentration of myricetin of 6.0g / L, molar ratio of myricetin raw material drug to HP-β-CD of 1:1, and solution volume flow rate of 1.0mL / min, the CO 2The effect on the morphology and recovery of myricetin inclusion compound when the flow rate is 2.0-2.5, 2.5-3.0, 3.0-3.5, 3.5-4.0, 4.0-4.5L / min respectively. Among them, when the flow rate is ≤2.5L / min, the product is similar to the rotary evaporation product under the same conditions, and it is a yellow transparent sticky solid attached to the bottom of the crystallization kettle; whe...
Embodiment 2
[0051] Example 2: Application of supercritical antisolvent method to prepare myricetin / HP-β-CD inclusion complex ultrafine particles
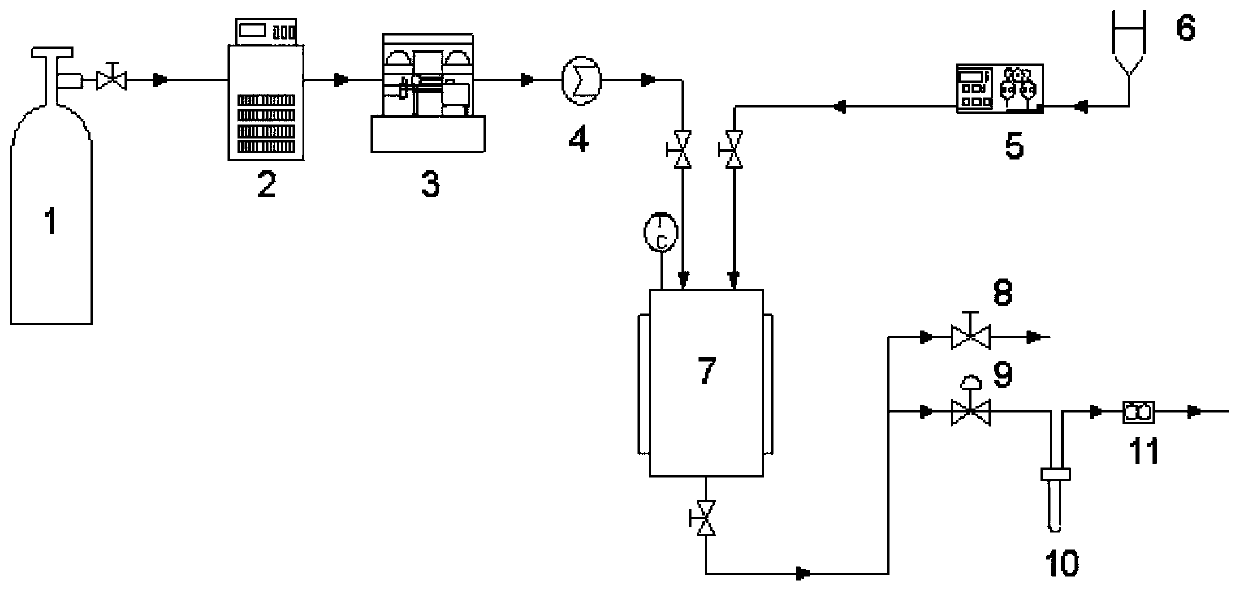
[0052] The method for preparing myricetin / HP-β-CD clathrate ultrafine particles by supercritical antisolvent method comprises the following steps:
[0053] Step S1, dissolving the myricetin raw material drug and the water-soluble carrier in an organic solvent to obtain a myricetin-carrier mixed solution;
[0054] Step S2, the CO 2 Pass into the crystallization kettle, adjust the temperature and pressure in the crystallization kettle;
[0055] Step S3, continue to feed CO 2 , maintaining the temperature and pressure in the crystallization kettle constant, while passing the myricetin-carrier mixed solution prepared in step S1 into the crystallization kettle;
[0056] Step S4, after the mixed solution is passed through, continue to pass through CO 2 40min, after exhausting the residual solvent, release the pressure; when the pressure in the cr...
Embodiment 3
[0066] Embodiment 3: In vitro dissolution test
[0067] Take a certain amount of myricetin raw material drug, myricetin / HP-β-CD solid mixture and myricetin / HP-β-CD inclusion compound ultrafine particles under the optimal process respectively, and measure their concentration in 0.1% Tween 80 The dissolution rate of the solution bell, compare its dissolution performance, the results are as follows Figure 9 As shown, the analysis shows that the dissolution rate of myricetin / HP-β-CD inclusion compound ultrafine particles can reach 85.69% in 5 minutes, and can reach more than 99% in 60 minutes, which proves that the dissolution of the drug has been significantly improved.
[0068] The in vitro dissolution results show that, compared with the raw material drug and solid mixture, the dissolution performance of myricetin / HP-β-CD inclusion complex ultrafine particles has been significantly improved, specifically, myricetin / HP-β-CD inclusion complex The cumulative dissolution rate of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com