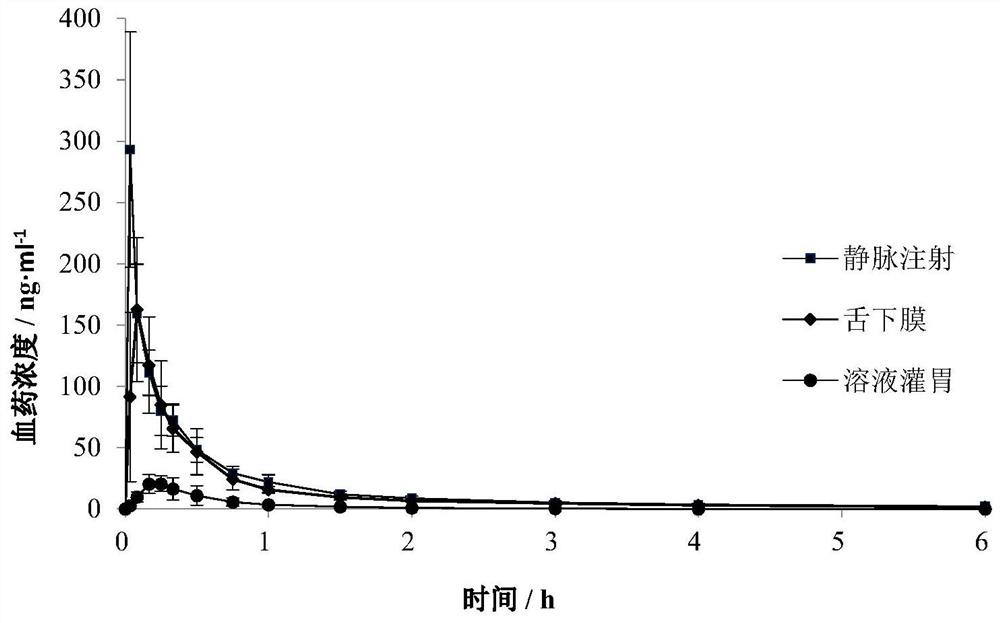
Rasagiline or medicinal salt sublingual film agent thereof as well as preparation method and application thereof
A technology for medicinal salts and film preparations, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as poor stability and increased content of oxidized impurities, and achieve good stability. , Rapid dissolution and good compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
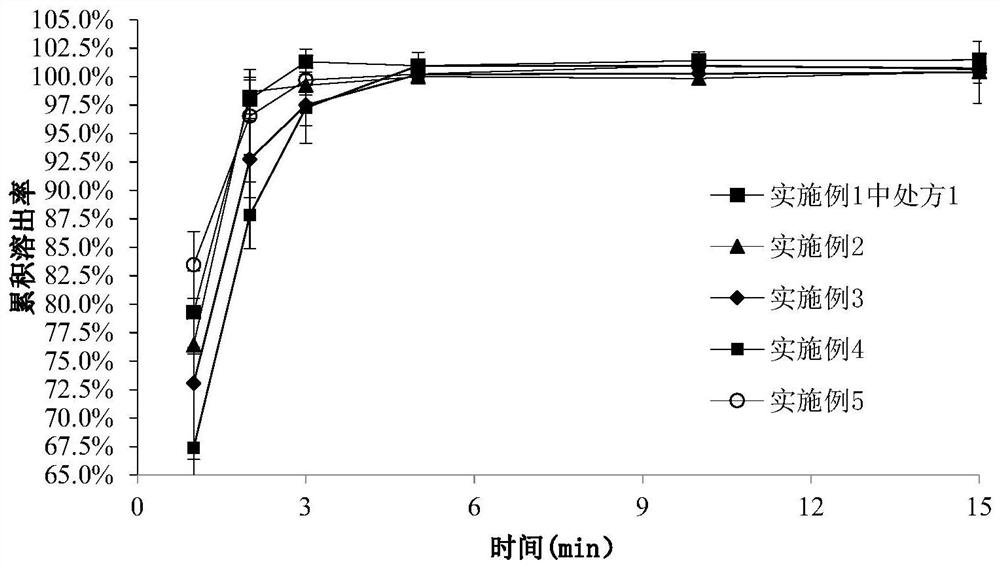
[0061] The compositions and dosages of prescriptions 1-5 and comparative prescriptions are shown in Table 1.
[0062] Table 1
[0063]
[0064] Preparation Process:
[0065] 1. Prepare the water slurry of the film-forming material: Dissolve HPMC and PVP in water according to the ratio of the prescription to prepare a water slurry with a mass concentration of 25%.
[0066] 2. Add rasagiline mesylate, hydroxypropyl-β-cyclodextrin, steviol glycoside, titanium dioxide and strawberry essence into the above water slurry, and stir evenly.
[0067] 3. Stand still for defoaming, and apply the film under the condition that the thickness of the film is 0.4mm and the drying temperature is 85°C. The speed of coating and film forming is 50cm / min.
[0068] 4. According to the specification of 0.5mg / tablet, it is cut and packaged.
[0069] It can be seen from Table 1 that (1) the physical properties of the film of prescription 1-3 of the present invention are better in strength and toug...
Embodiment 2
[0071] The prescription of embodiment 2 is as shown in table 2.
[0072] Table 2
[0073] Name of raw material Mass % Rasagiline mesylate 5 Hydroxypropyl Cellulose HPC 40 Sodium Carboxymethyl Cellulose CMC-Na 30 Maltodextrin 5 Glucosyl-β-cyclodextrin 5 Maltitol 10 Titanium dioxide 4 grape essence 1 total 100
[0074] Preparation Process:
[0075] 1. Prepare the water slurry of the film-forming material: 40g of HPC and 30g of CMC-Na are dissolved in water to prepare a water slurry with a mass concentration of about 20%.
[0076] 2. Add rasagiline mesylate, maltodextrin, glucosyl-β-cyclodextrin, maltitol, titanium dioxide, and grape essence into the above water slurry, and stir evenly.
[0077] 3. Stand still for defoaming, and apply the film under the condition that the thickness of the film is 0.4mm and the drying temperature is 85°C. The speed of coating and film forming is 50cm / min.
[0078] 4. Accordin...
Embodiment 3
[0080] The prescription of embodiment 3 is as shown in table 3.
[0081] table 3
[0082] Name of raw material Mass % Rasagiline mesylate 8 Polyvinyl alcohol PVA 30 corn starch 30 Maltodextrin 20 Aspartan 5 Titanium dioxide 4 Sweet Orange Flavor 3 total 100
[0083] Preparation Process:
[0084] 1. Prepare the water slurry of the film-forming material: 30g of PVA and 30g of cornstarch are dissolved in water to prepare a water slurry with a mass concentration of about 16%.
[0085] 2. Add rasagiline mesylate, maltodextrin, aspartame, titanium dioxide, and sweet orange essence into the above water slurry, and stir evenly.
[0086] 3. Stand still for defoaming, and apply the film under the condition that the thickness of the film is 0.4mm and the drying temperature is 85°C. The speed of coating and film forming is 50cm / min.
[0087] 4. According to the specification of 0.5mg / tablet, it is cut and packaged.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com