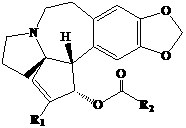
Oral solid pharmaceutical composition in micronized form and preparation method thereof
A composition and micronization technology, applied in the field of medicine, can solve the problems of severe side effects, poor bioavailability, incompleteness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
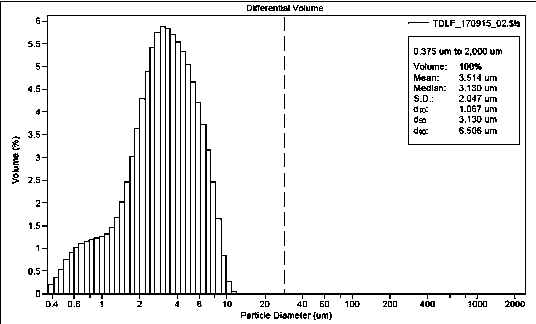
[0037] The homoharringtonin derivatives were ultrafinely pulverized using MQP01 airflow pulverizer, and pulverized into D 90 Fine powder below 10μm, crushing conditions: low-temperature drying air temperature is 6°C, water content is 0.5%, inlet pressure is 0.8MPa, working pressure of ultrafine pulverizer is 0.8MPa, internal working temperature is 6°C, crushing times for 3 times.
Embodiment 2
[0039] Use a jet mill to ultrafinely pulverize the homoharringtonin derivative raw material, collect the pulverized raw material particles, and use a Beckman LS 13 320 XR laser diffraction particle size analyzer to measure the particle size of the fine powder, and the particle size at 95% cumulative volume The micronized homoharringtonin derivative is obtained below 10 μm.
Embodiment 3
[0040] Embodiment 3 Take the solid pharmaceutical composition prescription 1~4
[0041] Raw materials (g) / 1000 pieces Prescription 1 Prescription 2 Prescription 3 Prescription 4 Comparative example 1 Homoharringtonin derivatives 50 15 50 50 50 (not pulverized, particle size above 80μm) Hydroxypropyl Cellulose 30 30 45 15 30 lactose monohydrate 20 30 10 30 20 microcrystalline cellulose 35 60 30 40 35 Croscarmellose Sodium 10 10 10 10 10 Sodium dodecyl sulfate 3 3 3 3 3 Micropowder silica gel 2 2 2 2 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com