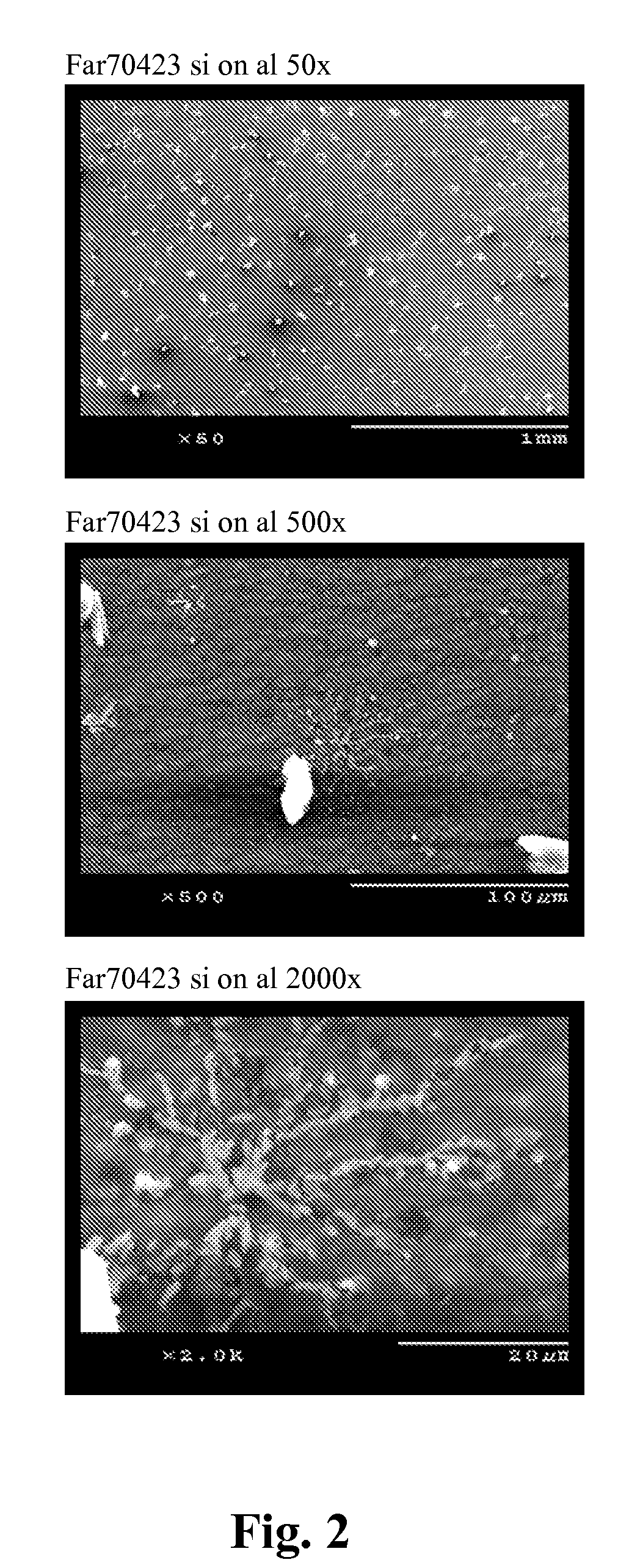
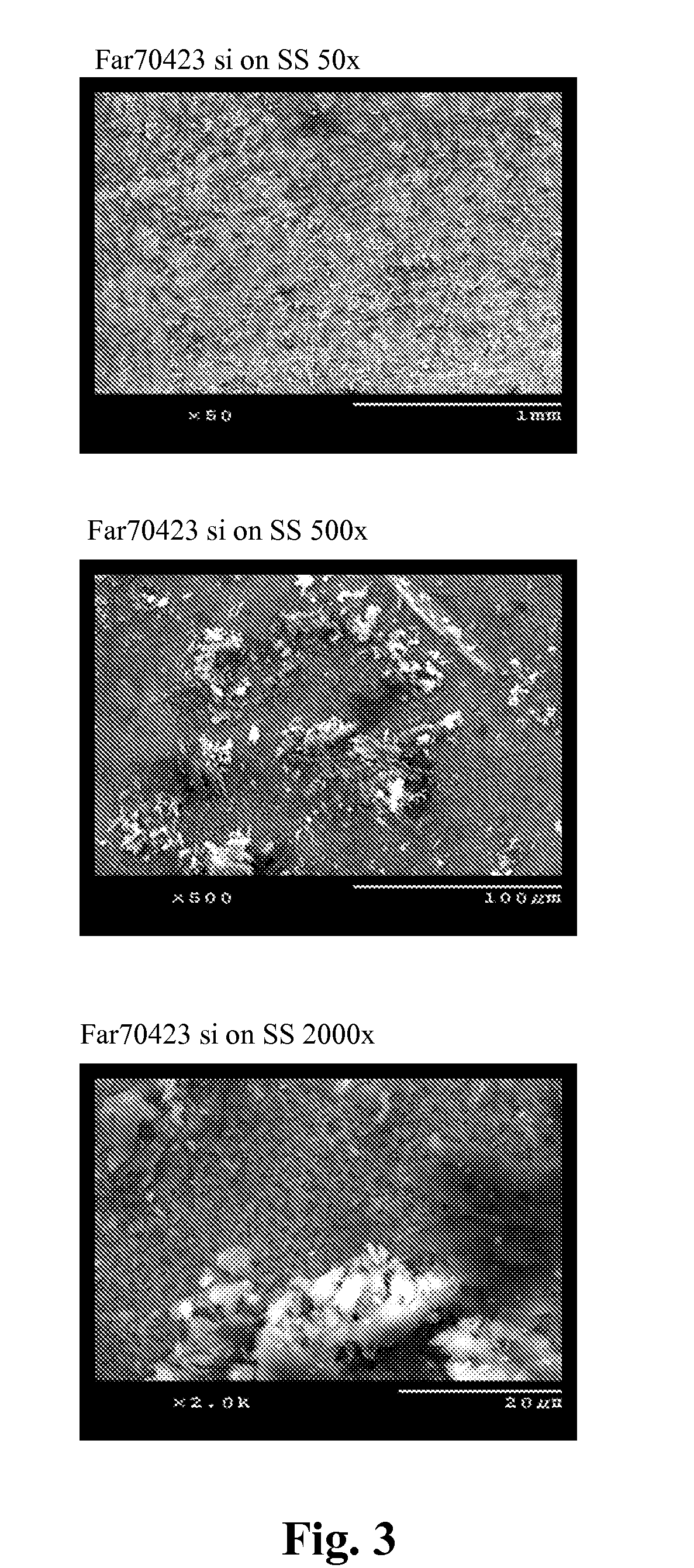
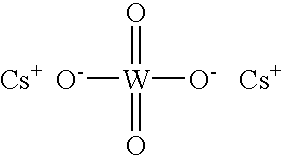Patents
Literature
322 results about "Metal sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sulfates are salts or esters of sulfuric acid, H 2 SO 4, formed by replacing one or both of the hydrogen atoms with a metal cation or organic group. Most metal sulfates are readily soluble in water, but calcium sulfate is only slightly soluble, while barium, lead, and strontium sulfates are insoluble.
Reactive compositions for fluid treatment
InactiveUS6861002B2Easy to disassembleImprove efficiencyPerfluorocarbons/hydrofluorocarbons captureLoose filtering material filtersCystFiltration
A method and device for the chemical conversion, filtration and / or purification of fluids water or other solutions containing microbiological and chemical contaminants, such as fluids containing arsenic, chlorine, bacteria, viruses, and cysts, where the fluid is passed through a purification material composed of fluid treatment carbon, metal phosphates, metal oxides, reduced metals, metal silicates, metal sulfates, metal carbonates, metal hydroxides, or combinations thereof. The material may be included in a fixed binder matrix.
Owner:WATERVISIONS INT
Reactive compositions for fluid treatment
InactiveUS20030196966A1Perfluorocarbons/hydrofluorocarbons captureLoose filtering material filtersFiltrationPhosphate
A method and device for the chemical conversion, filtration and / or purification of fluids water or other solutions containing microbiological and chemical contaminants, such as fluids containing arsenic, chlorine, bacteria, viruses, and cysts, where the fluid is passed through a purification material composed of fluid treatment carbon, metal phosphates, metal oxides, reduced metals, metal silicates, metal sulfates, metal carbonates, metal hydroxides, or combinations thereof. The material may be included in a fixed binder matrix.
Owner:WATERVISIONS INT
Positive electrodes for lithium batteries
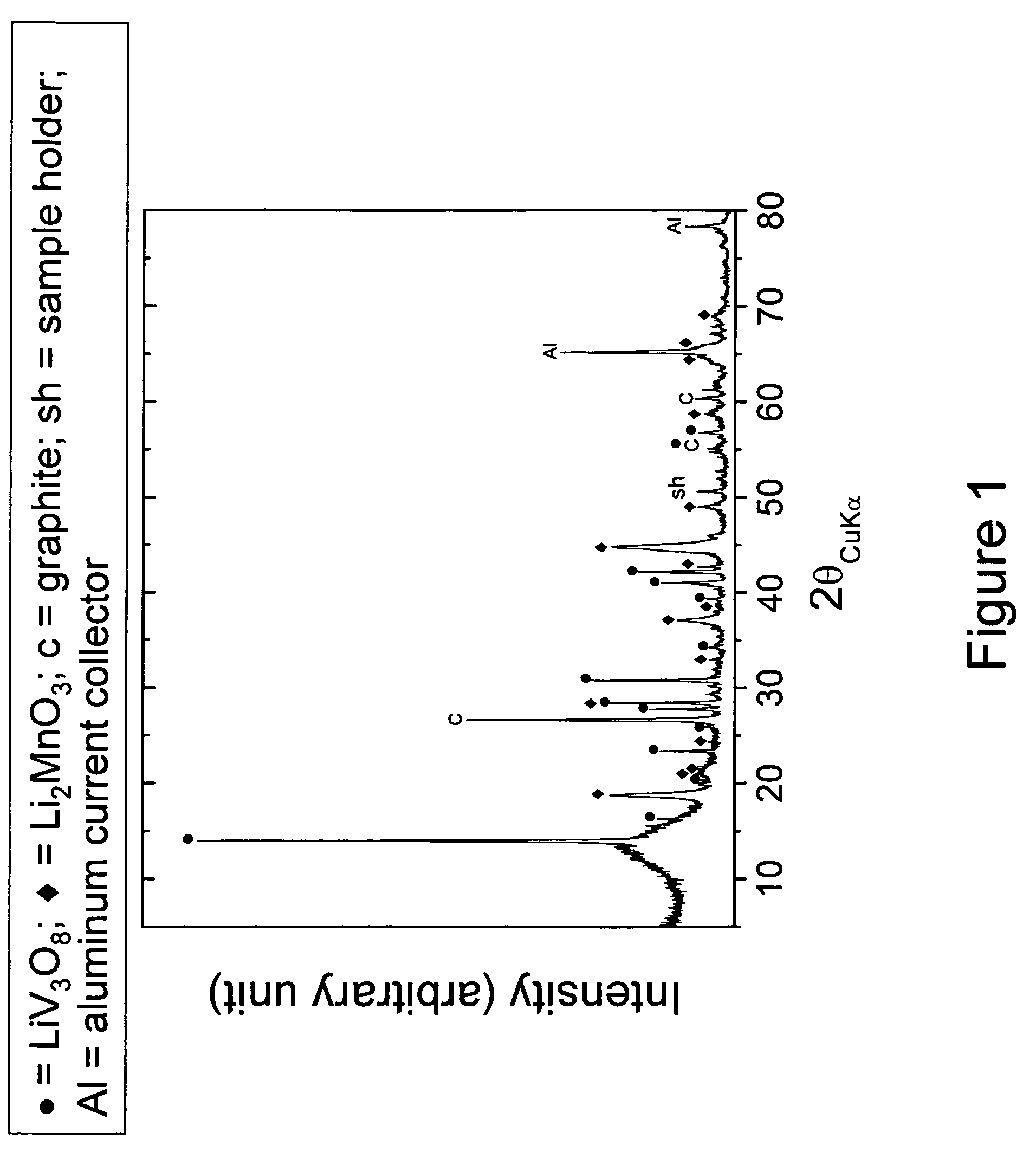
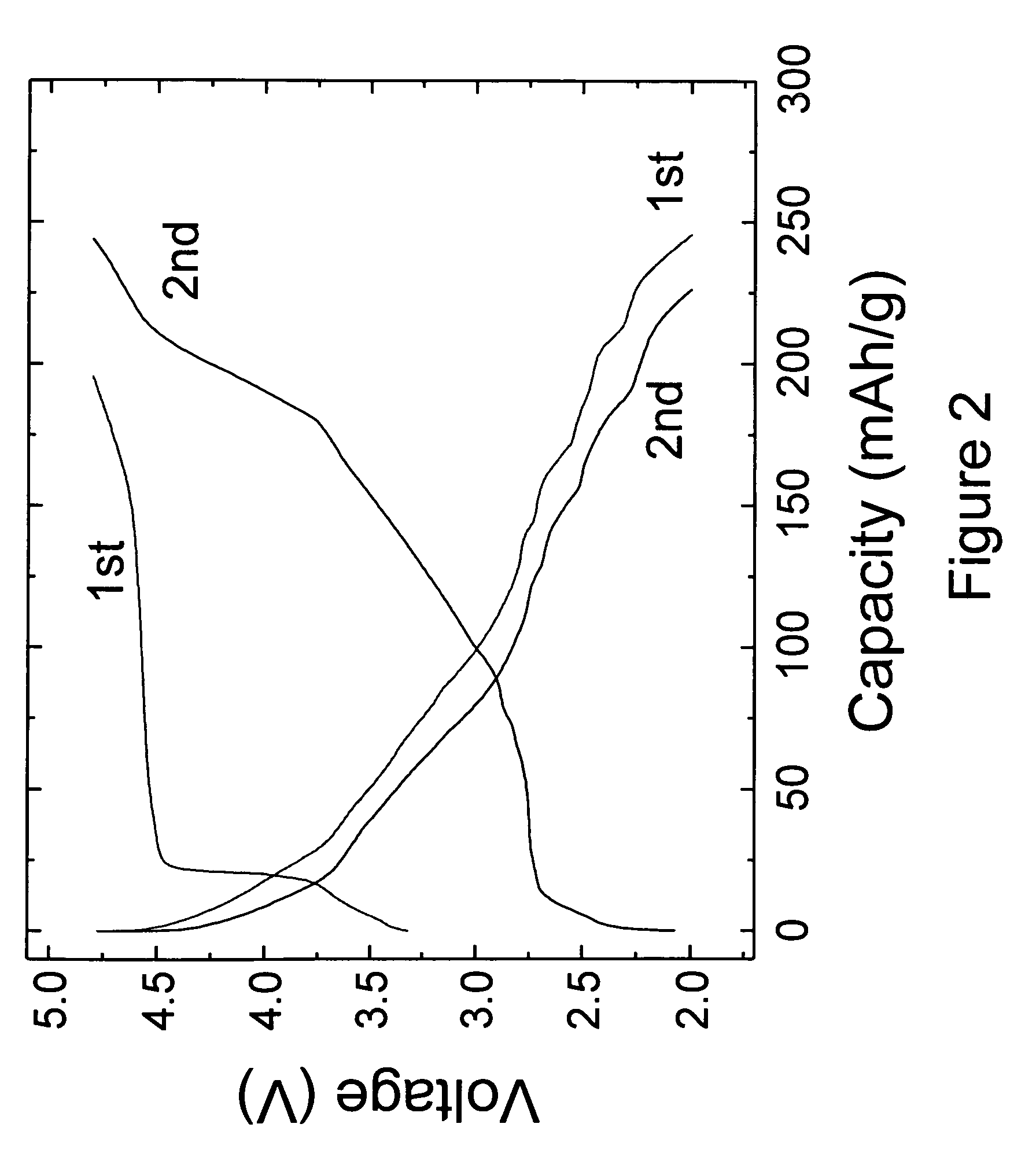
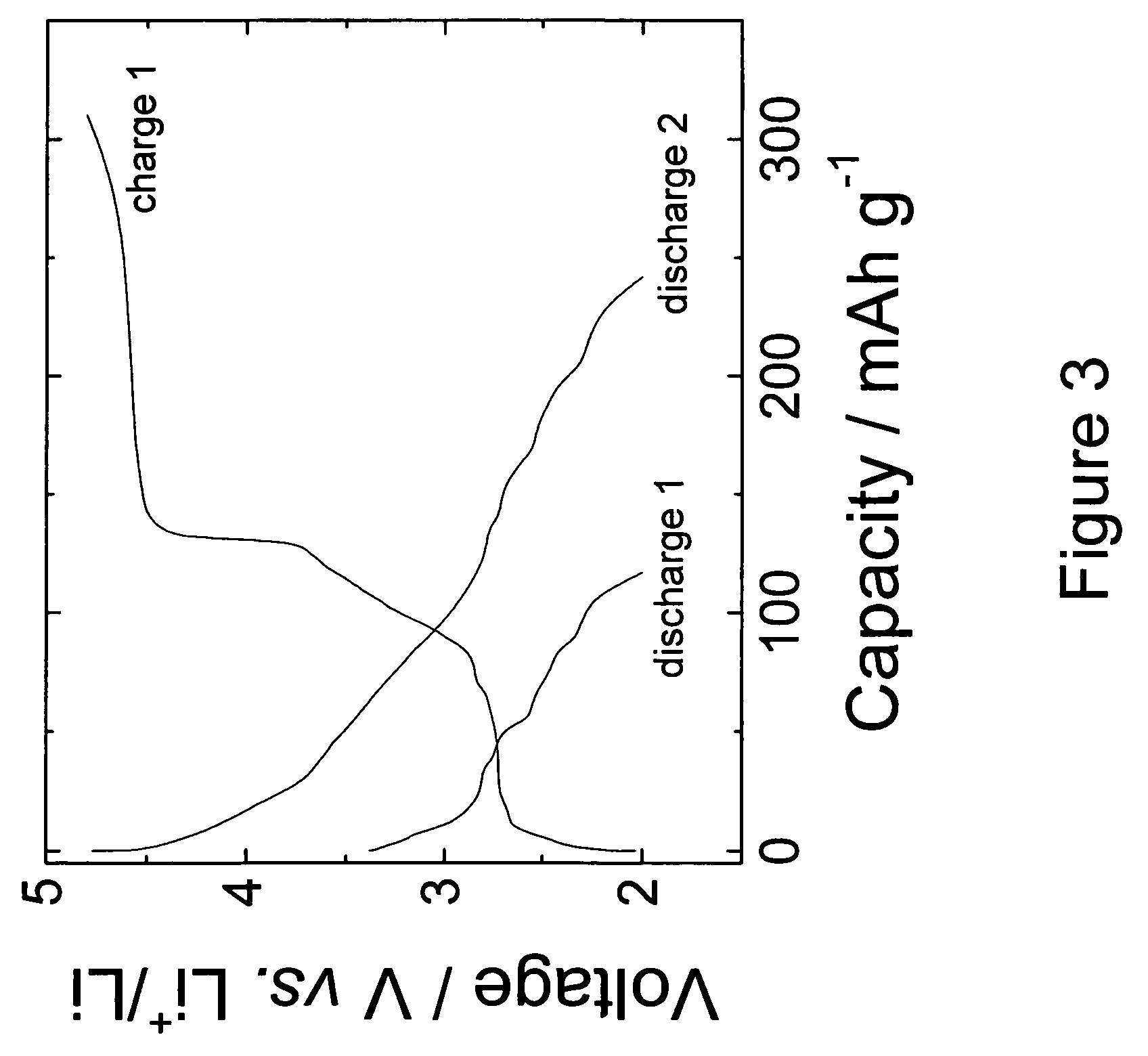
This invention provides lithium-rich compounds as precursors for positive electrodes for lithium cells and batteries. The precursors comprise a Li2O-containing compound as one component, and a second charged or partially-charged component, selected preferably from a metal oxide, a lithium-metal-oxide, a metal phosphate or metal sulfate compound. Li2O is extracted from the above-mentioned electrode precursors to activate the electrode either by electrochemical methods or by chemical methods. The invention also extends to methods for synthesizing and activating the precursor electrodes and to cells and batteries containing such electrodes.
Owner:UCHICAGO ARGONNE LLC
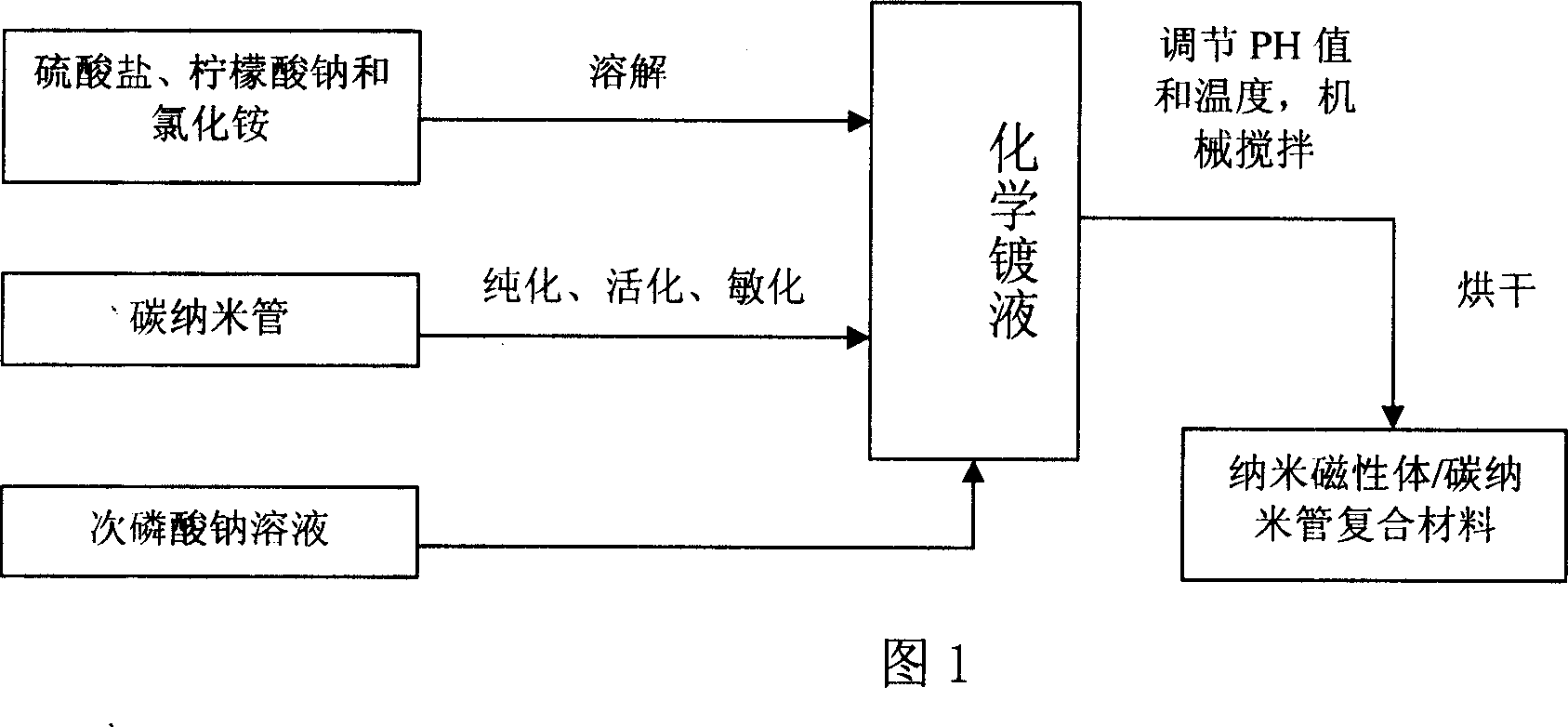
Carbon nano tube wave absorbtion mateirla of surface carried with magnetic alloy particle and preparation method thereof
InactiveCN101045533AGood electrical and magnetic propertiesSimple preparation processNanostructure manufactureScreening apparatusMicrowaveCarbon nanotube
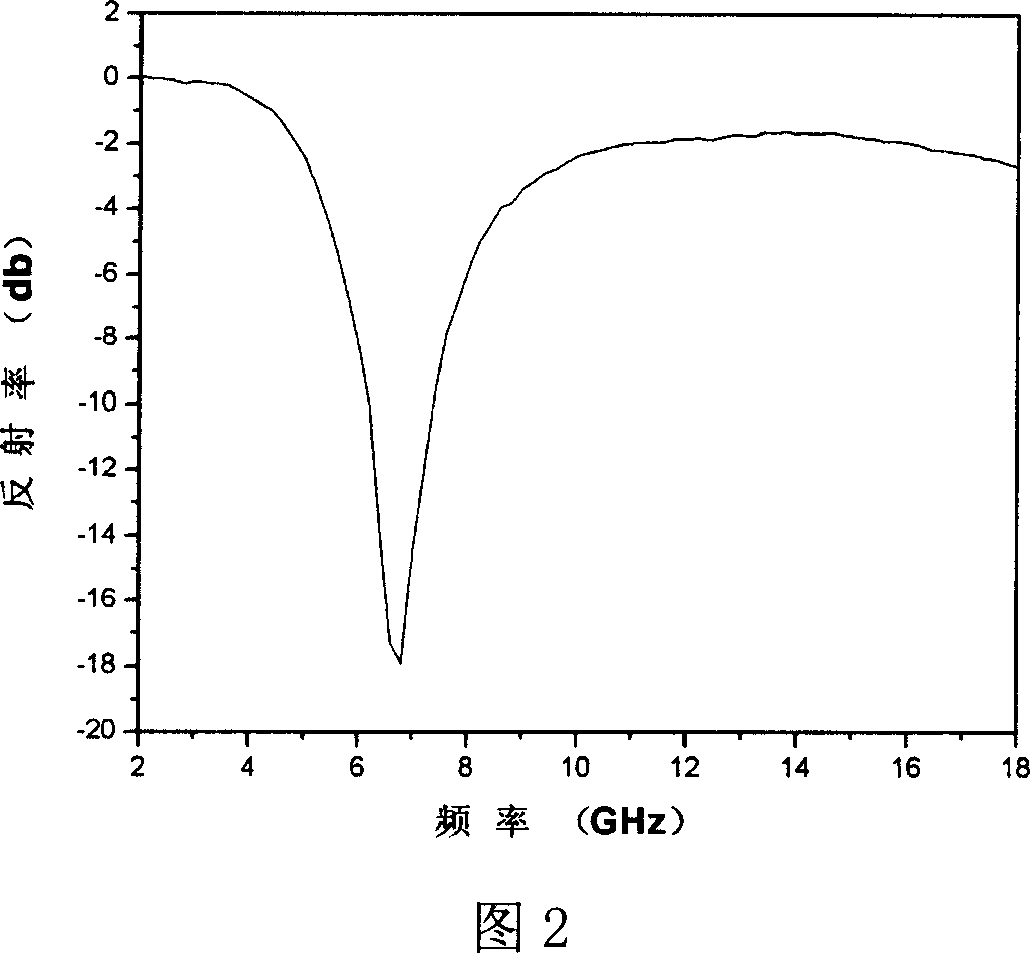
A carbon nanotube carrying magnetic alloy particles on its surface and used as wave absorbing material is prepared through uniformly dispersing carbon nanotubes in the solution of the sulfate of Fe, Co and Ni, and oxidizing-reducing reaction for deposition the magnetic alloy particles on carbon nanotubes.
Owner:TSINGHUA UNIV
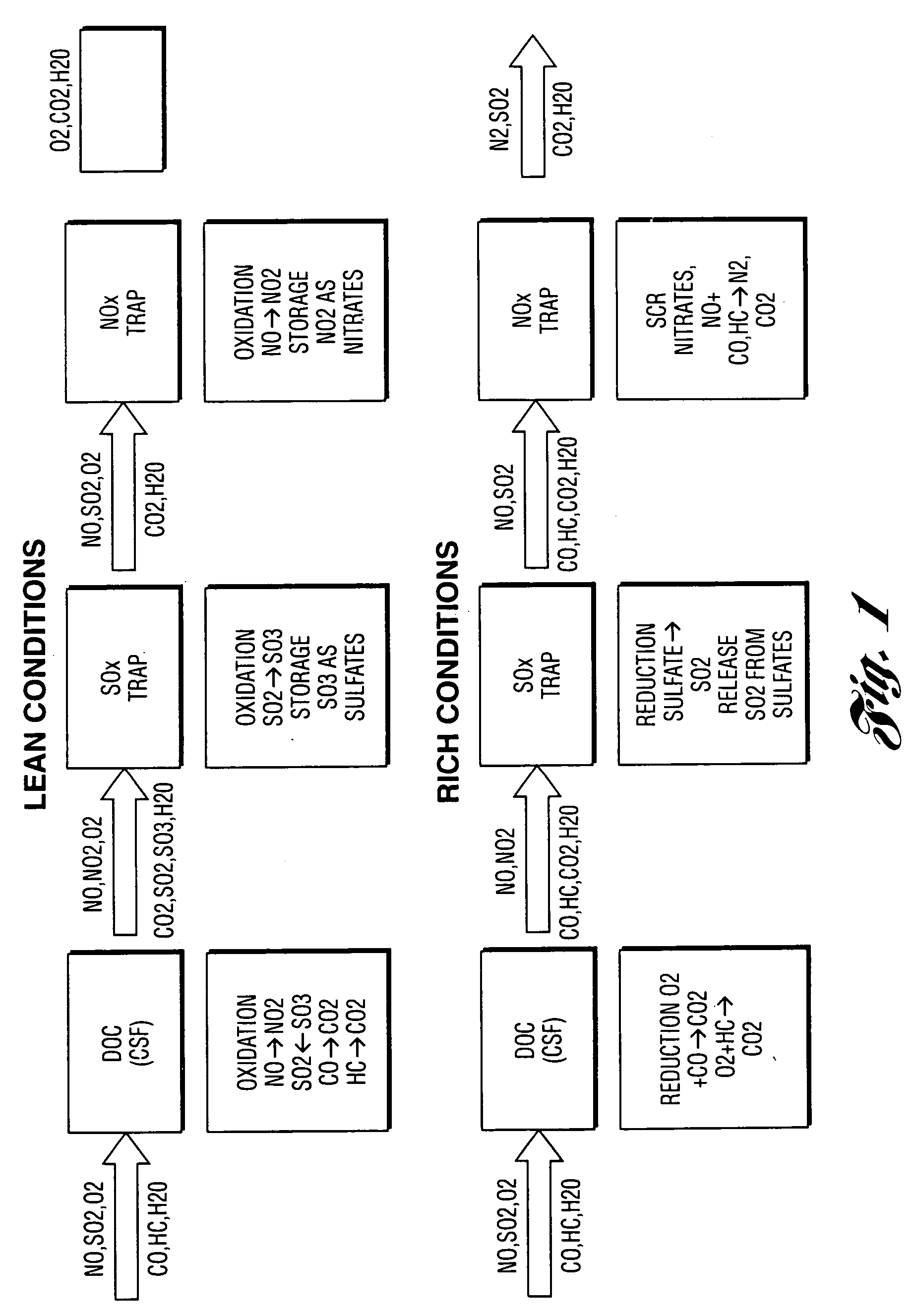
SOx trap for diesel and lean-burn gasoline automotive applications
InactiveUS20050145827A1Reduce gas emissionsAvoid problemsCombination devicesGas treatmentSulfurGasoline
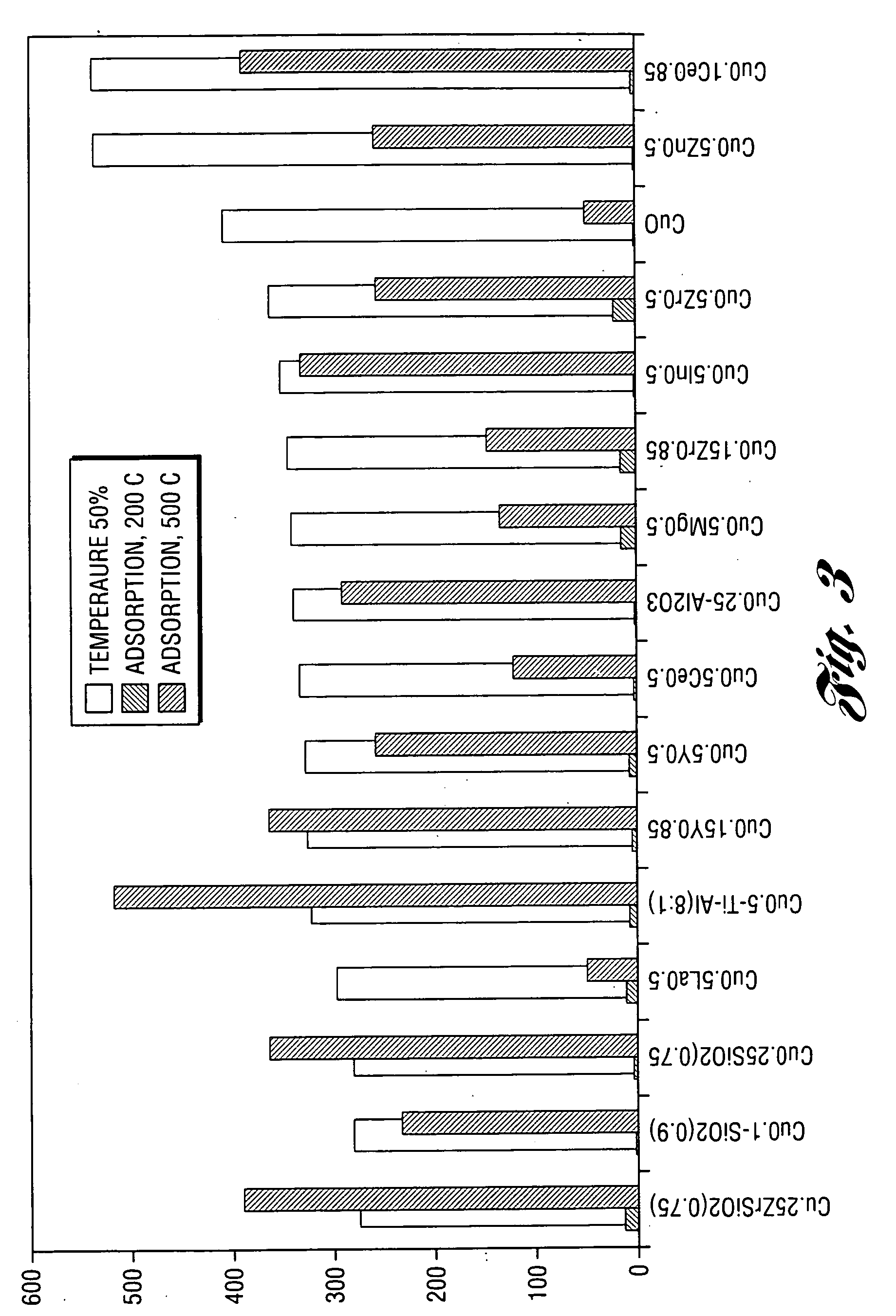
The present invention provides a regenerable catalyst composition suitable for entrapping SOx. The composition of the invention comprises a copper oxide having the formula (Cu / (A oxide) where A oxide is SiO2, Zr—SiO2, A12O3, TiO2—Al2O3, ZrO2 and In2O3 or mixtures thereof. Copper loading may vary from about 10 to 60 mol % and is preferably about 25 mol %. The catalyst composition adsorbs SOx as metal sulfate under lean conditions and desorbs accumulated SOx as SO2 under rich conditions. Such reversible SOx trap are able to operate under conventional NOx trap operating conditions to prevent sulfur poisoning of the NOx trap. Furthermore, these traps may be regenerated under rich conditions at 300-450° C. In another embodiment of the present invention, an irreversible SOx trap capable of collecting SOx under lean conditions is provided. The traps of this embodiment include praseodymia, zirconia-praseodymia and mixed manganese-yttria and mixtures thereof.
Owner:FORD GLOBAL TECH LLC
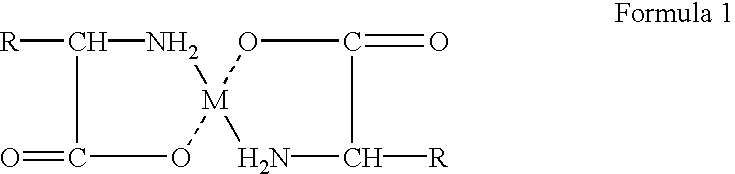
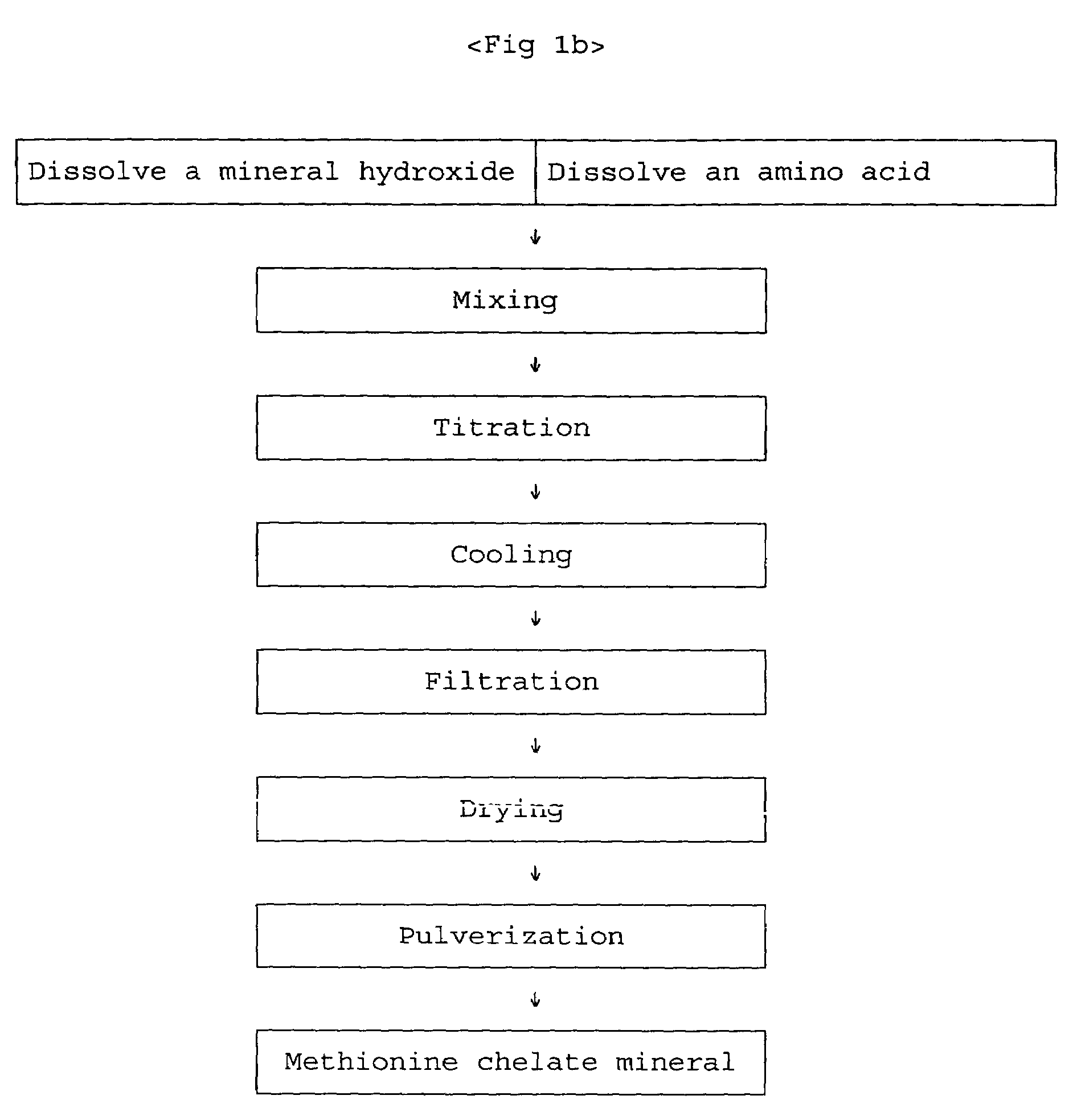
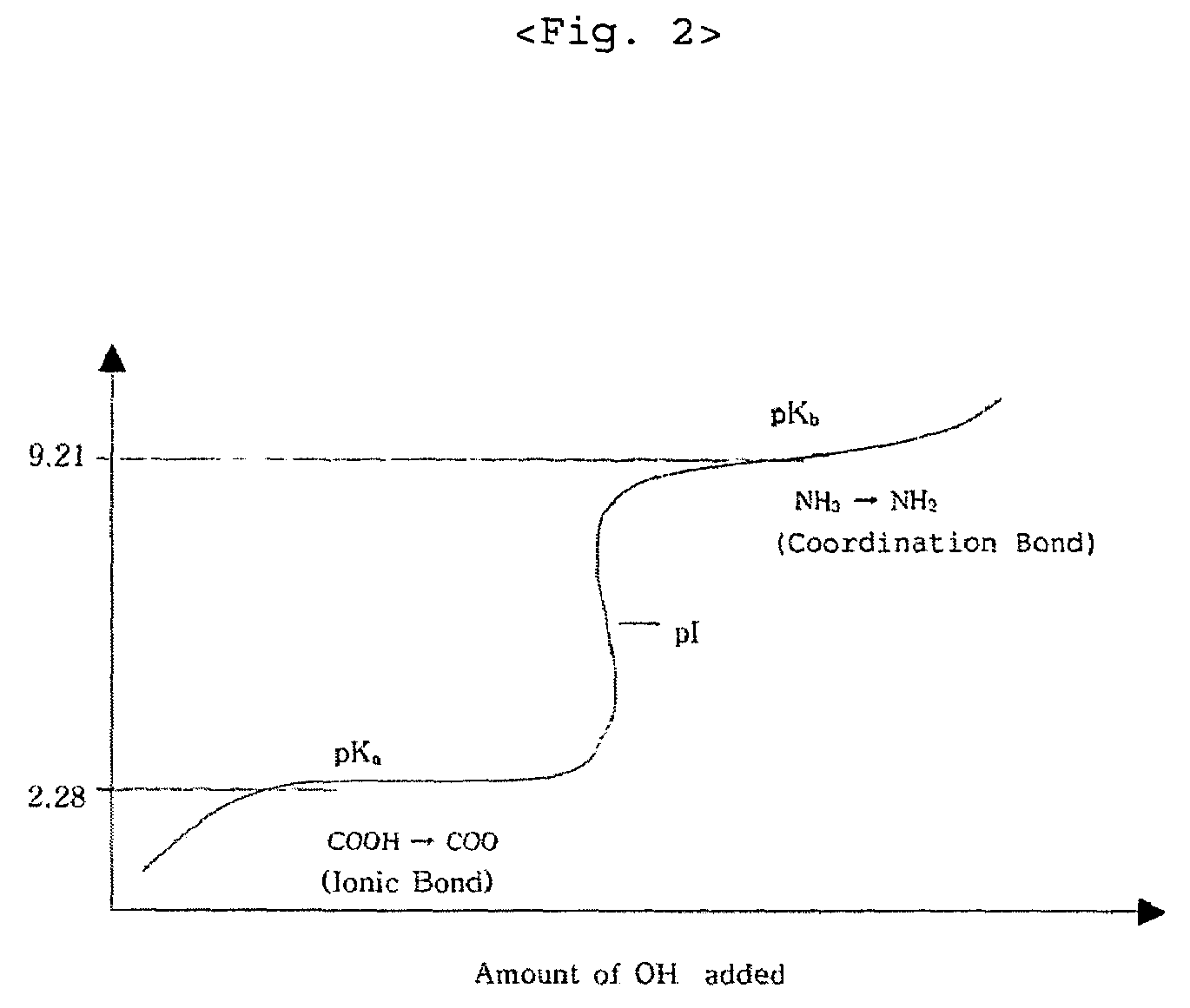
Composition and method for preparing amino acid chelates and complexes
Compositions and methods of preparing amino acid chelates and complexes are disclosed and described. Specifically, by (a) combining a hydrated metal sulfate salt with an amino acid ligand to form a particulate blend, (b) placing the particulate blend in an enclosed environment; and (c) applying heat to the particulate blend in the enclosed environment causing the waters of hydration of the metal sulfate salt to be released into the enclosed environment causing a reaction resulting in the formation of an amino acid chelate or complex by effecting the reaction between functional electron rich groups of the amino acid ligand and a metal ion of the metal sulfate salt. The waters of hydration serve to provide the water necessary to enable a bonding reaction to take place between the electron rich functional groups of the amino acid ligand and the metal ion of the hydrated metal sulfate salt.
Owner:ALBION LAB
Liquid and solid effluent treatment process
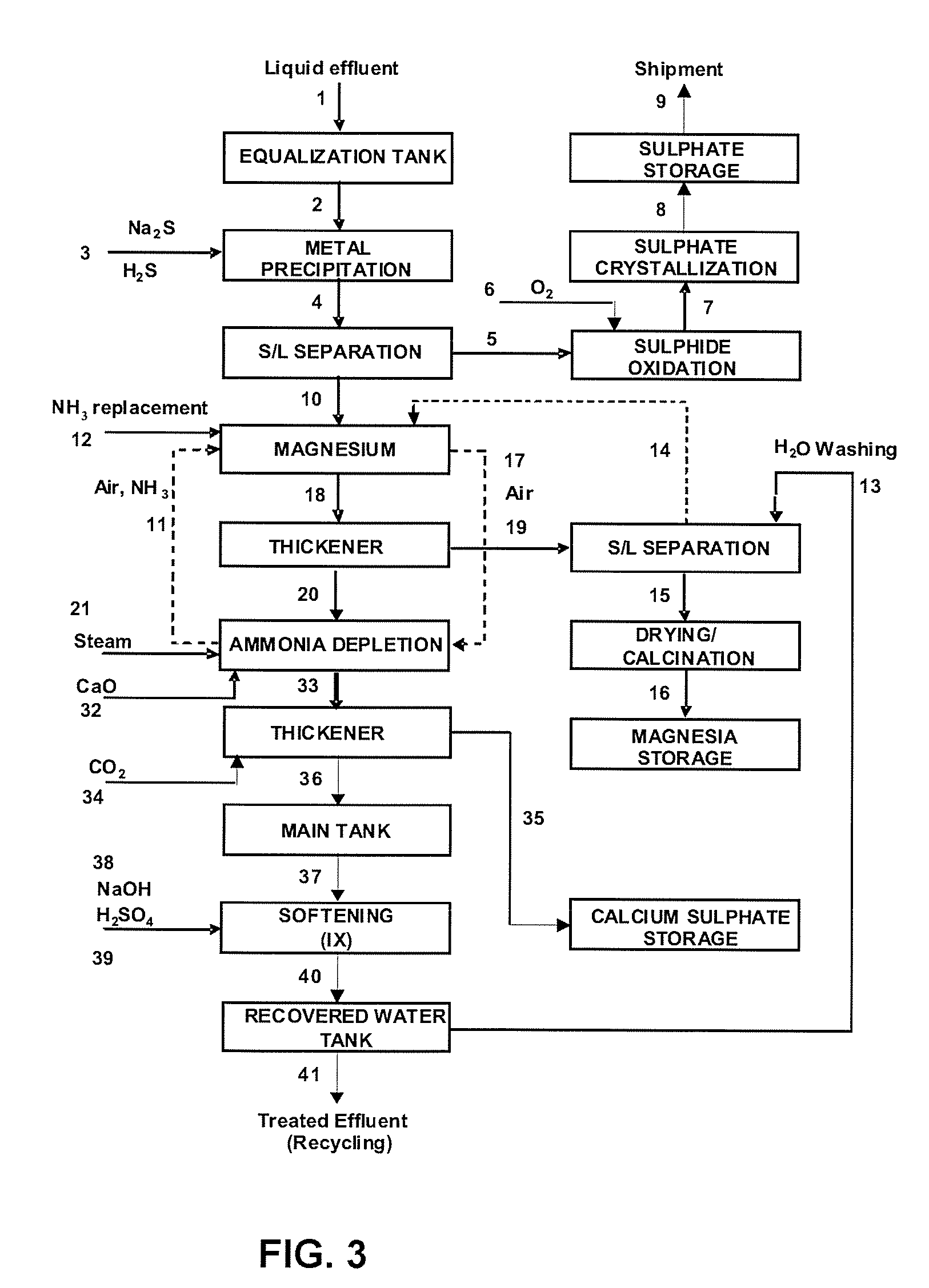
ActiveUS20090180945A1Reduce consumptionReduce environmental impactCobalt ammonia complexesNitrogen compoundsAmmonium sulfateChemistry
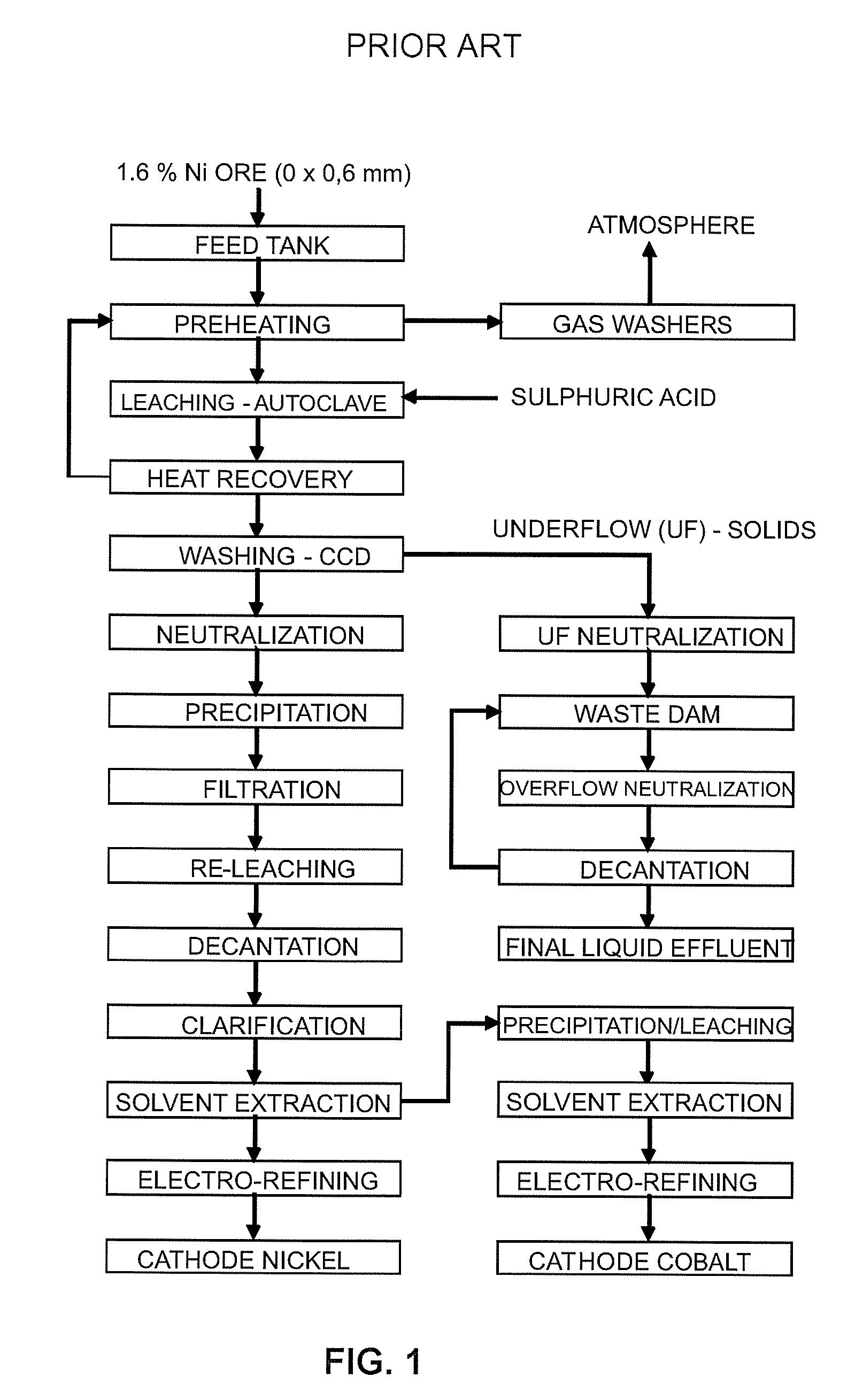
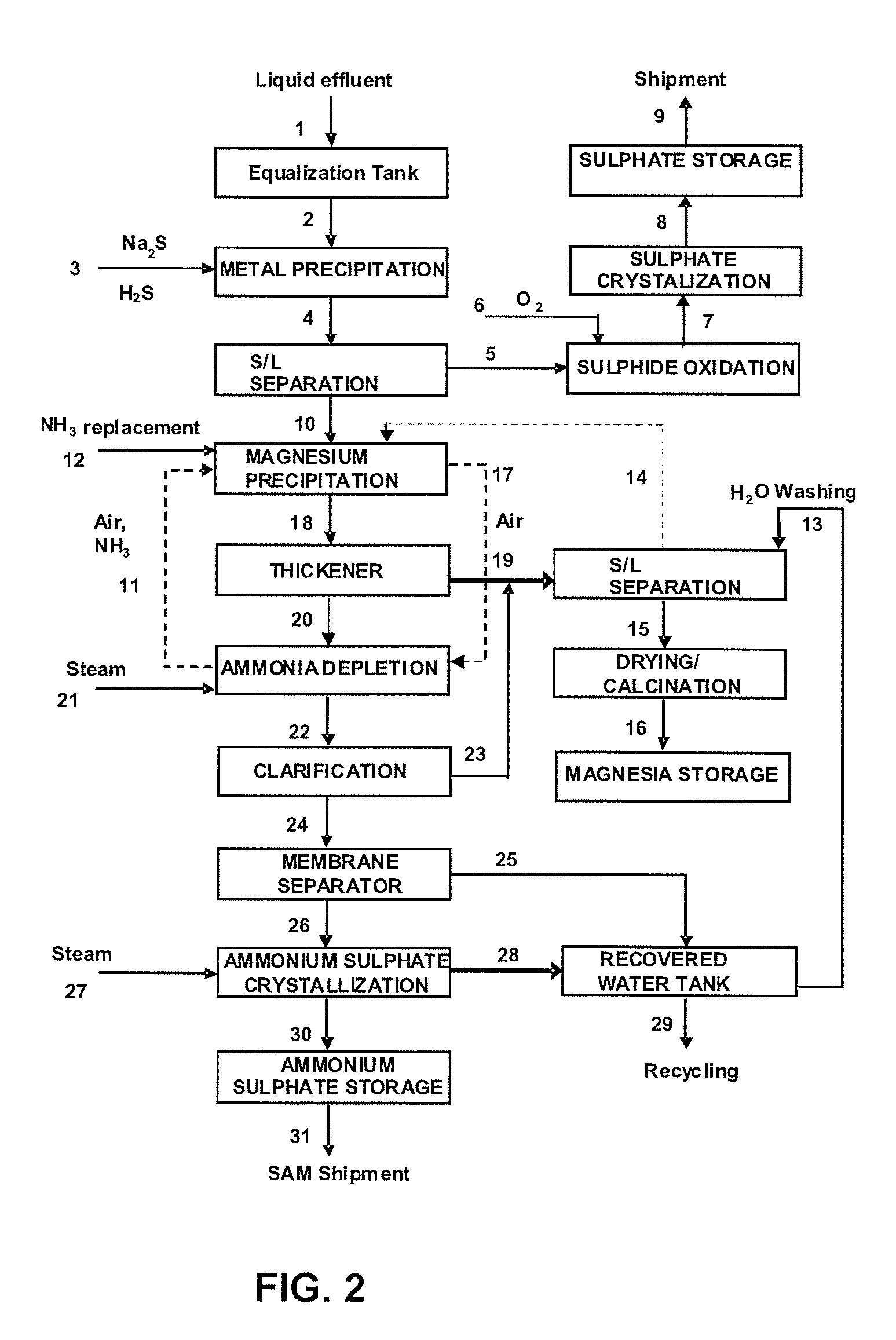
A process for recovering metals such as Ni, Co, Mn, Cu, Zn, among others, through precipitation as sulphides, enabling recovery of magnesium in the form of hydroxide, carbonate and oxide and providing recovery of sulphate as gypsum and ammonium sulphate. Liquid phase, after full treatment, comprises recovered water with a quality proper for total reuse in industrial process.This process of liquid and solid effluent treatment is provided with flexibility to process several types of effluents presenting wide variations in their chemical composition. The main steps of this process are: (1) equalization of liquid effluent, (2) precipitation of metals as sulphides, (3) oxidation of metallic sulphides and crystallization as metallic sulphates, (4) precipitation of magnesium as hydroxide and calcination thereof into magnesium oxide (5) recovery of ammonia, (6) preconcentration of the remaining saline solution, (7) evaporation / crystallization of ammonium sulphate, (8) storage of recovered water, (9) partial or total precipitation of sulphate contained in the effluent with quicklime, (10) segregated storage of gypsum and gypsum-magnesium mixture, (11) softening of the remaining solution and (12) storage of softening water.
Owner:VALE CANADA
Acidic composition of matter for use to destroy microorganisms
A composition of matter and the method of making that provide a low pH acidic composition that is useful for destroying microorganisms that are undesirable and useful for destroying or reducing melanoma on human skin. The composition and method include a strong, low pH acid combined with distilled water and urea or an ammonium compound, such as ammonium sulfate or other metallic sulfates, including but not limited to, sodium sulfate, magnesium sulfate, zinc sulfate, manganese sulfate, and copper sulfate, under at least 15 psi pressure in pressurized container, all of which is heated to approximately 800° F. or more for at least 3 hours. The final cooled mixture is stabilized with 10 to 15 percent of the original mixture. The resultant composition is useful for preserving food, such as fresh fish, and for skin treatment of melanoma and as bactericides, fungicides, or viricides.
Owner:CMS TECH
Novel nanoscale solution method for synthesizing lithium cathode active materials
InactiveUS20110300442A1Li-accumulatorsNon-aqueous electrolyte accumulator electrodesManganeseElectrochemistry
The present invention relates to a solution based method for preparing an nano scale electroactive metal polyanion or a mixed metal polyanion comprising reacting metal sulfate—M(SO4)x and / or other soluble metal salts, here M could be iron, cobalt, manganese, nickel or mixtures thereof, with a solution of sodium hydroxide with addition of solution of ammonium hydroxide, in the presence of water, drying the nano-intermediate M(OH)2 or M1M2(OH)2 or M1M2M3(OH)2, or MO(OH) or M1M2O(OH) or M1M2M3O(OH), mixing the dried intermediate with a soluble lithium precursor and soluble PO4 containing precursor and a soluble polymer carbon, well mixed the mixture, and then removing said solvent at a temperature and for a time sufficient to remove the solvent and form an essentially dried mixture; and heating said mixture at a temperature and for a time sufficient to produce an electroactive metal polyanion or electroactive mixed metal polyanion. It is another object of the invention to provide electrochemically active materials produced by said methods. The electrochemically active materials so produced are useful in making electrodes and batteries.
Owner:INFINITY ENERGY HONG KONG
Method for preparation of organic chelate
InactiveUS7087775B2Copper organic compoundsIron organic compoundsIntestinal structureHigh absorption
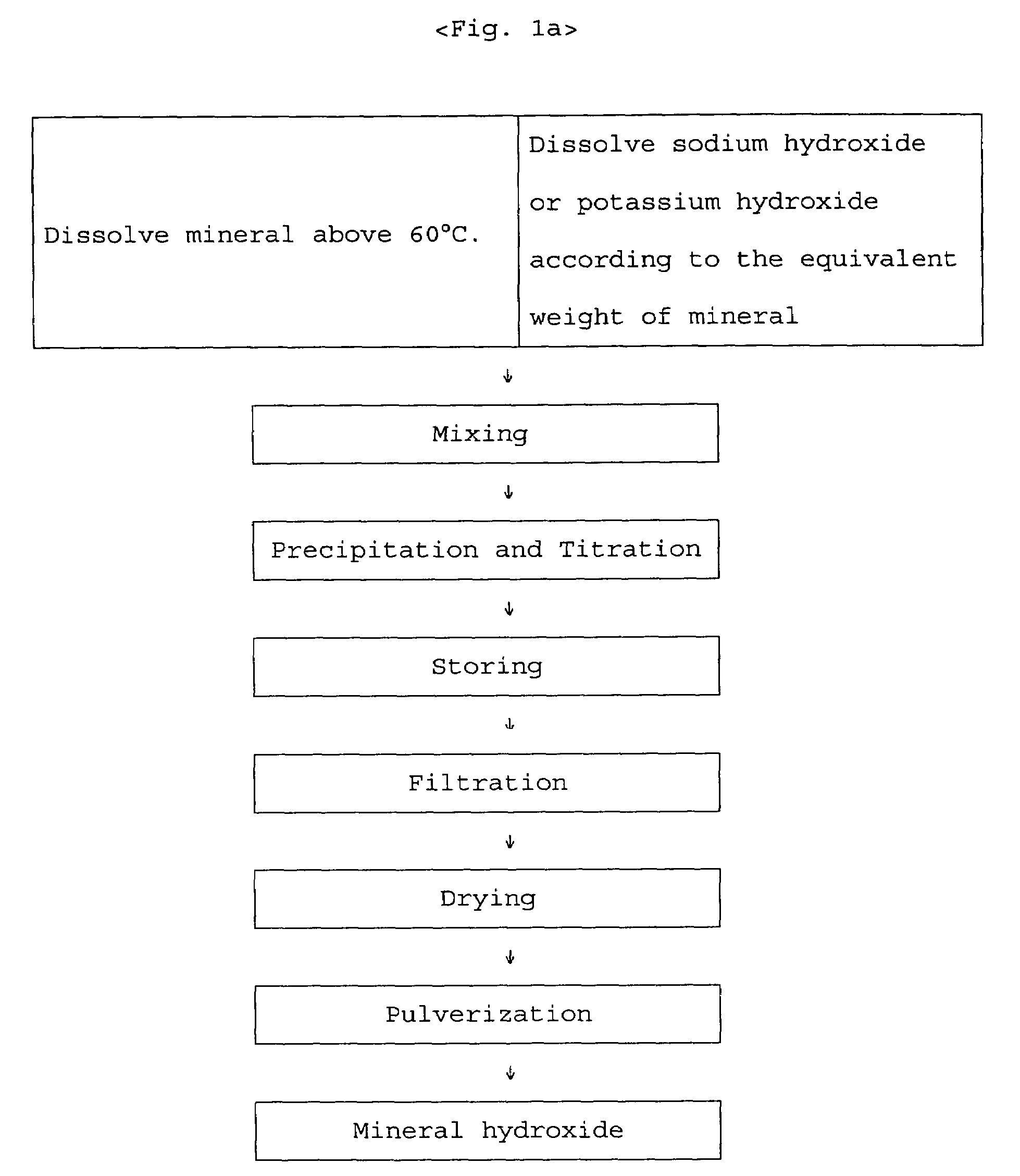
A method for the preparation of organic chelates utilized as ingredients of animal feedstuffs. The organic chelates are passed to the intestines of livestock without decomposition in the stomach, and thereby have a high absorption rate. The present invention provides a preparation method of perfect chelate minerals having a high yield and containing copper, zinc, iron, manganese, or cobalt. The organic chelate mineral is prepared by precipitating metal sulfates or chlorides with sodium hydroxide or potassium hydroxide in a solution of two equivalents of an amino acid completely dissolved at above 70° C., collecting metal hydroxide by removing sodium sulfate, potassium sulfate, sodium chloride or potassium chloride through filtering, dissolving the metal hydroxide by adding the equivalent weights of hydrochloric acid, and then neutralizing the hydrochloric acid added for dissolving metal hydroxide.
Owner:LEE SANG BUM +1
Algaecide compositions and methods of removing algae
InactiveUS6291397B1TimeKeep for a long timeBiocideDead animal preservationLithium hydroxidePotassium hydroxide
An algaecide formulated in a concentrated, solid composition form for subsequent dissolution in water is disclosed. The algaecide is designed to remove algae from a variety of interior and exterior surfaces. The preferred algaecide compositions chlorinated isocyanurates, such as sodium dichloro-s-triazinetrione, alkali hydroxides selected from the group consisting of sodium hydroxide, potassium hydroxide, calcium hydroxide, and lithium hydroxide; metal sulfates selected from the group consisting of copper sulfate, zinc sulfate, and aluminum sulfate; and a buffer.
Owner:WILKINS JR JOE S
Compositions and processes for deposition of metal ions onto surfaces of conductive substrates
InactiveUS20080302267A1Anti-corrosive paintsLiquid/solution decomposition chemical coatingPhosphateWater soluble
The present invention provides compositions and processes for preparing metallic ions for deposition on and / or into conductive substrates, such as metals, to substantially eliminate friction from metal to metal contact. It is used in the aqueous embodiment to form new metal surfaces on all metal substrates. The processes form stable aqueous solutions of metal and metalloid ions that can be adsorbed or absorbed on and / or into conductive substrates. The aqueous solutions consist of ammonium alkali metal phosphate salts, and / or ammonium alkali metal sulfate salts mixed with a water soluble metal or metalloid salt from Group I through Group VIII of the periodic table of elements. The aqueous solutions allow for a nano deposition of the metal ions on and / or into the surfaces of conductive substrates. The surfaces created by the deposited metal ions will provide metal passivation and substantially eliminate friction in metal-to-metal contact without the use of hydrocarbon based lubricants.
Owner:DFHS LLC
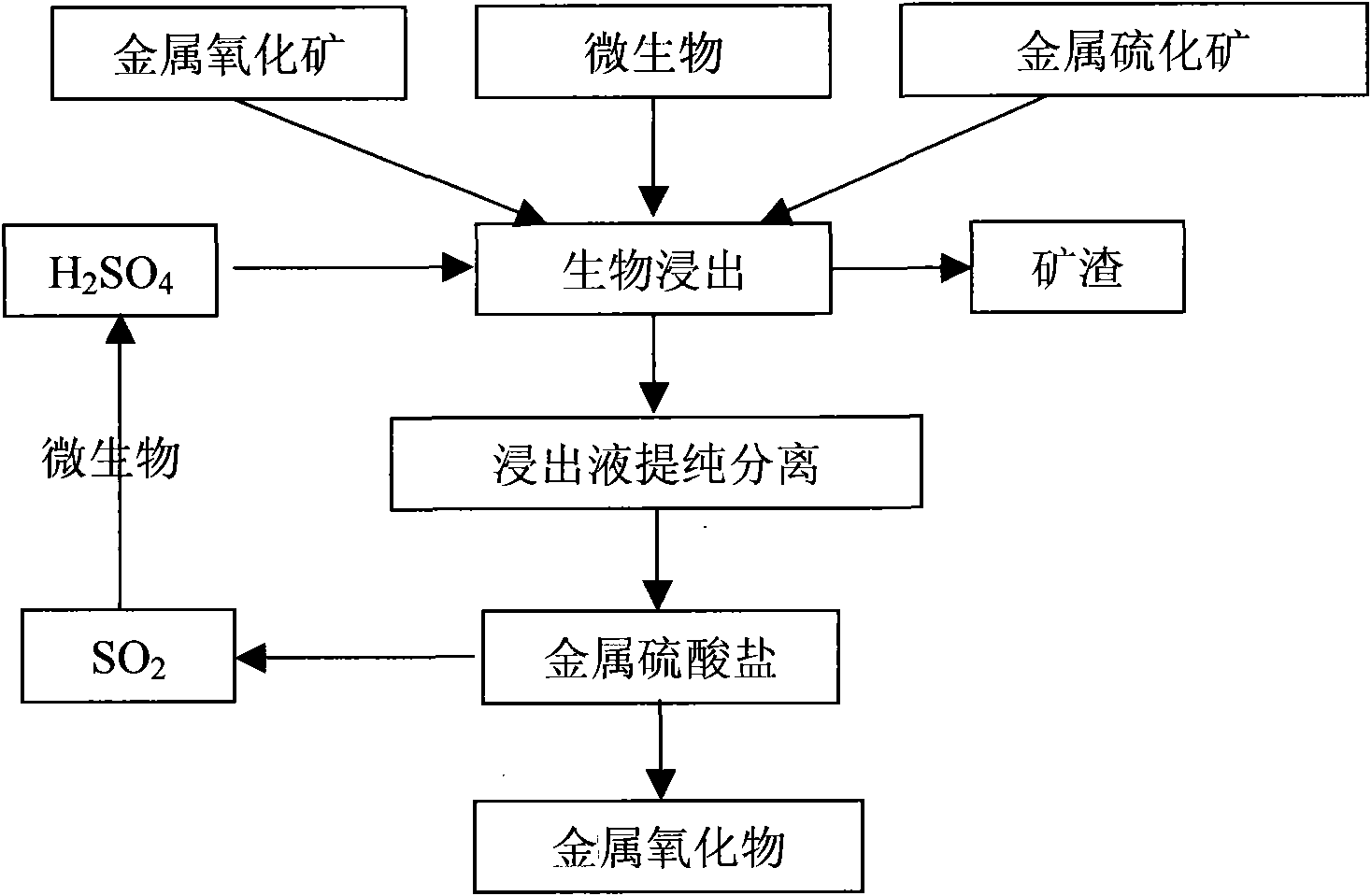
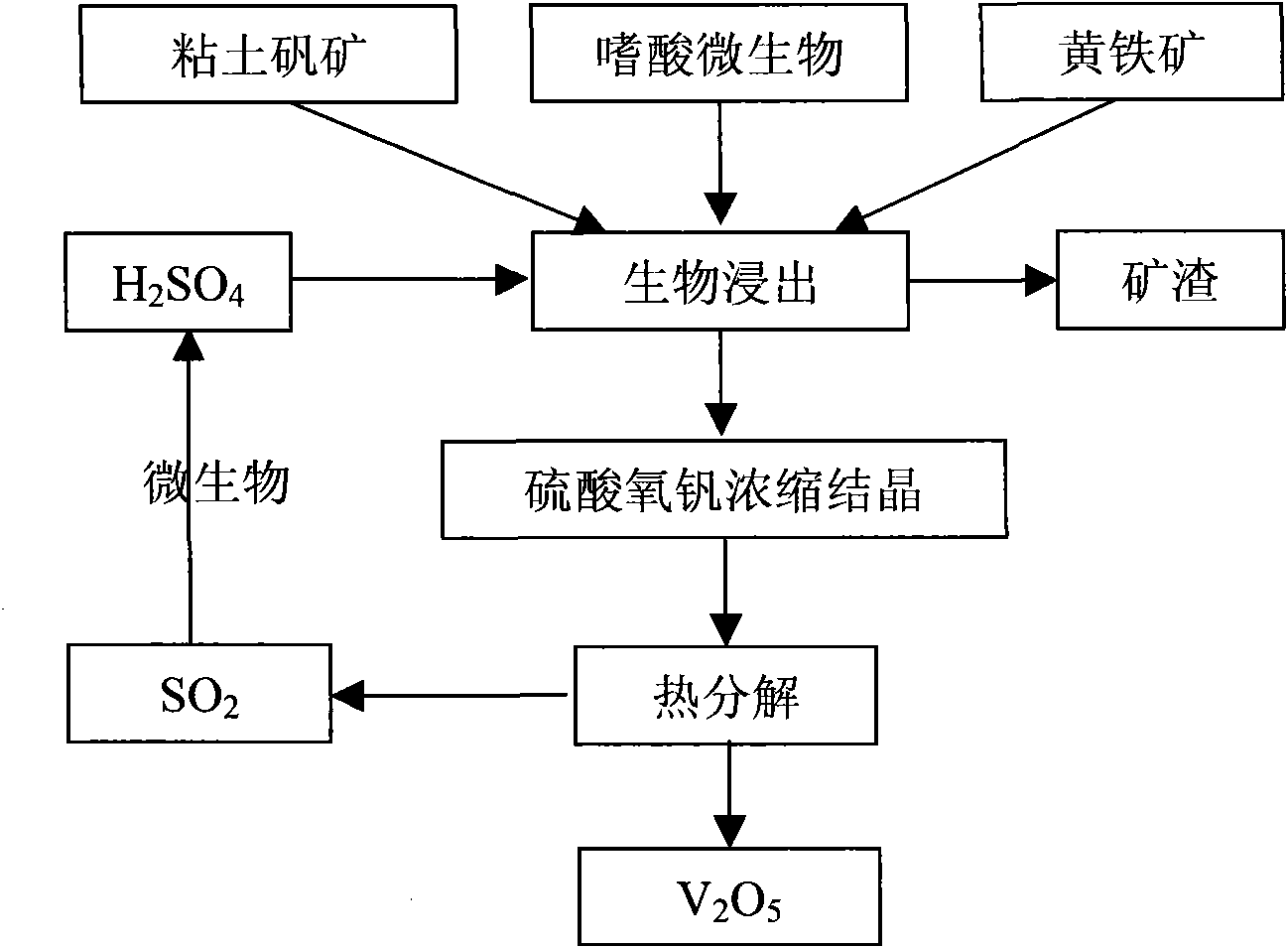
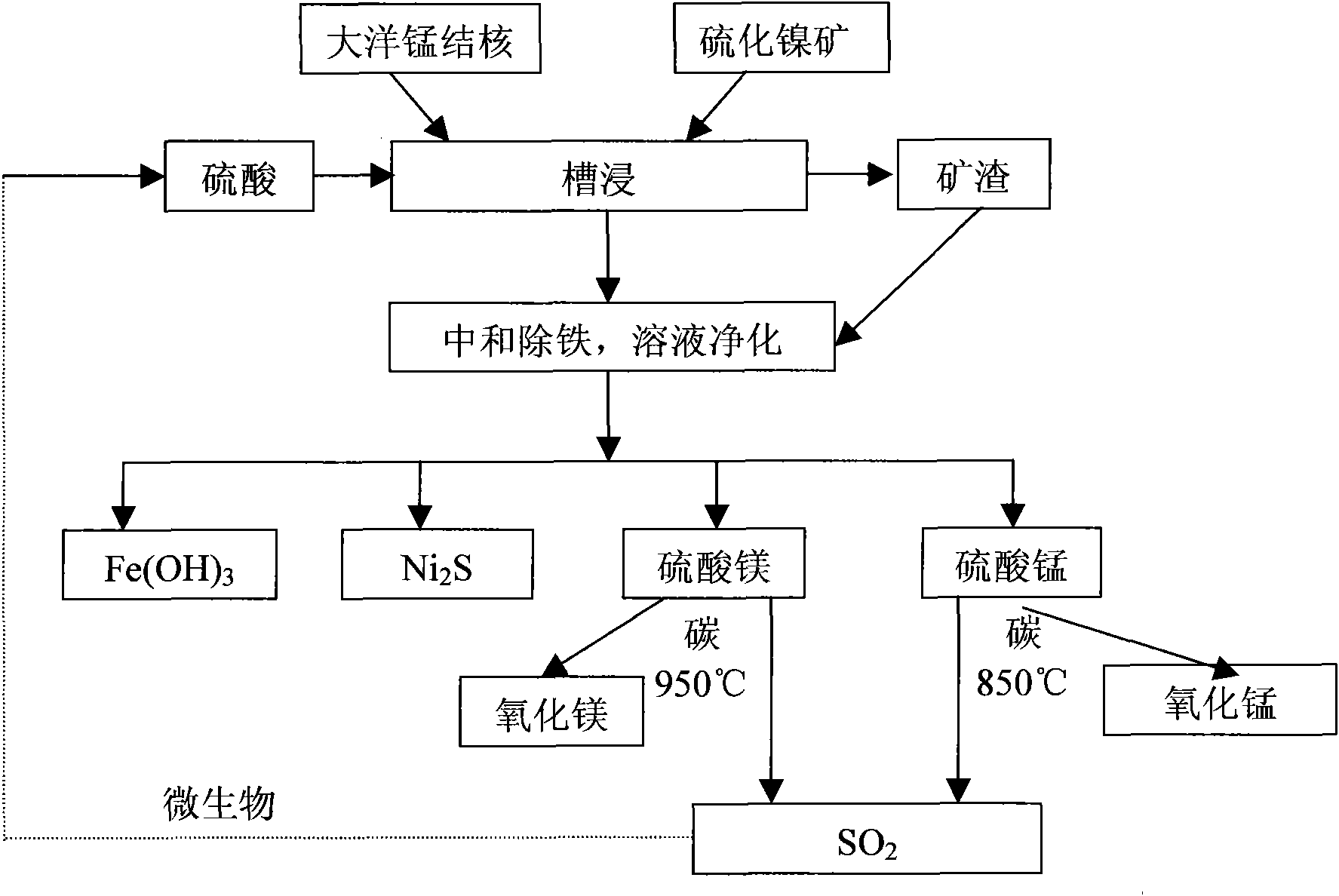
Biological-chemical metallurgy method by jointly utilizing oxidized ore and sulfide ore
InactiveCN101608260AHigh recovery rateUniversalProcess efficiency improvementMineral SourcesBiological oxidation
The invention belongs to the field of mineral resource comprehensive utilization, and relates to methods for reduced leaching of oxidized ore and oxidation leaching of sulfide ore through biological and chemical catalysis, in particular to a biological-chemical metallurgy method by jointly utilizing the oxidized ore and the sulfide ore. The reduced leaching of the metal oxidized ore and the oxidation leaching of the metal sulfide ore are realized by utilizing auxoautotrophs, auxohetertrophs or a mixture thereof under mild conditions; and the mixture ratio of the metal oxidized ore to the metal sulfide ore can be adjusted by adding organic biomass. The leachate is purified, concentrated and crystallized into metal sulfates which are then reduced and thermally decomposed into products of sulfur dioxide and metal oxides; and the sulfur dioxide is subjected to biological oxidation to prepare sulphuric acid for recycling.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Alkali metal tungstate compositions and uses thereof
ActiveUS20030114318A1Reduce the amount requiredReciprocating drilling machinesConstructionsAlkaline earth metalFormate
Fluids, such as completion fluids, containing at least one alkali metal tungstate and optionally at least one chelating agent are described. Methods of removing a filter cake from a well bore surface, which may include one or more alkaline earth metal sulfates, is also described, wherein the method includes contacting the filter cake with the completion fluid of the present invention. A drilling fluid or mud is also described wherein the drilling fluid contains at least one alkali metal tungstate. The drilling fluid preferably further contains at least one emulsifier or surfactant and at least one hydrocarbon-based fluid. The various fluids of the present invention can contain other conventional ingredients and optionally at least one alkali metal formate. The present invention permits the fluids to be essentially solids free due to the use of the alkali metal tungstate.
Owner:CABOT SPECIALTY FLUIDS
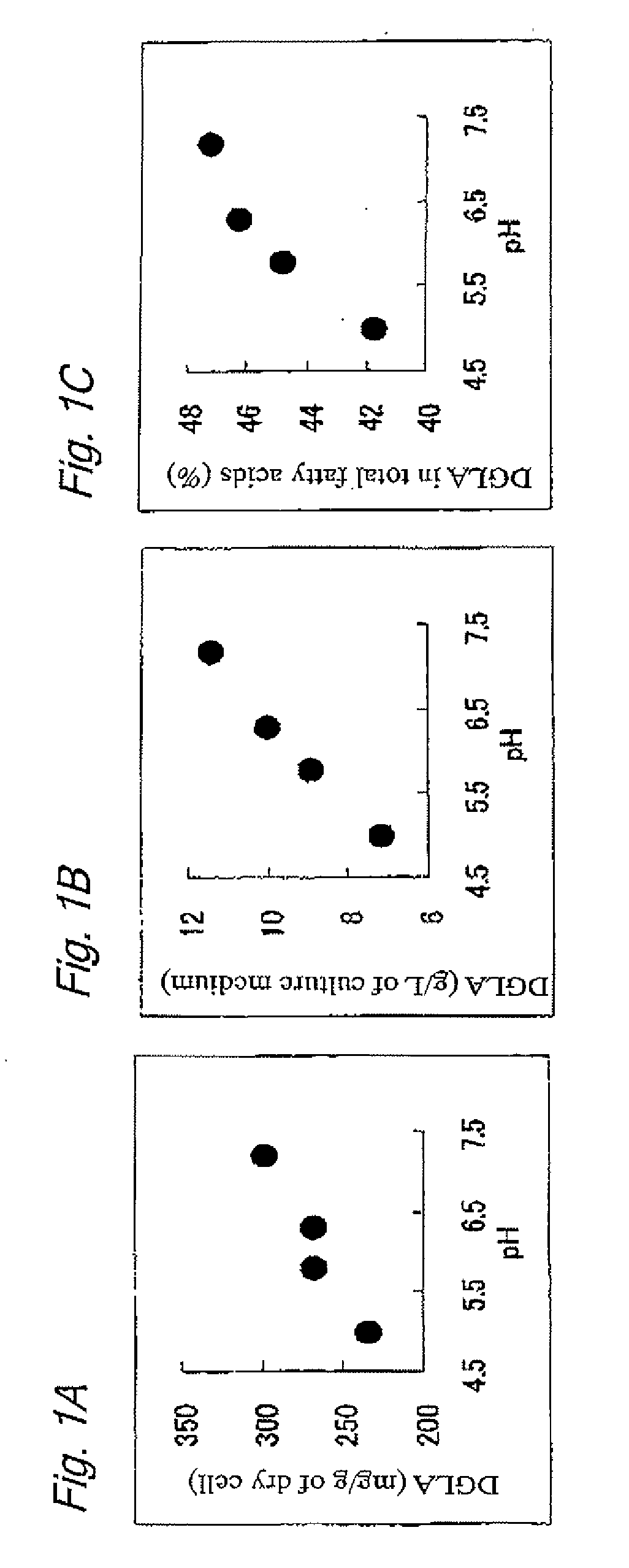
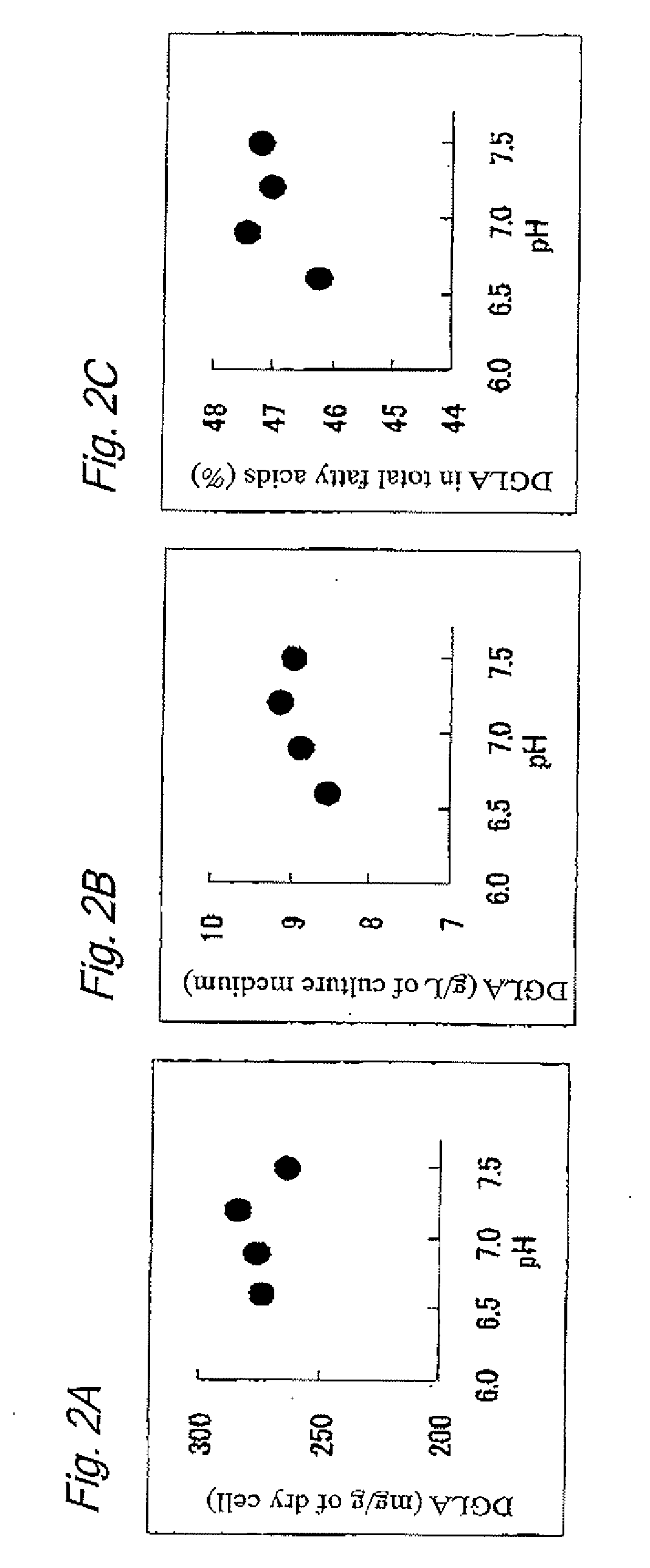
Methods for producing polyunsaturated fatty acid and lipid containing polyunsaturated fatty acid
ActiveUS20100167359A1Easy to handleIncrease contentFungiFatty-oils/fats refiningLipid formationOrganic acid
A method for producing a polyunsaturated fatty acid (PUFA) or a lipid containing a PUFA, a microbial cell containing a PUFA, and use of the microbial cell are provided. A method for producing a polyunsaturated fatty acid (PUFA) or a lipid containing a PUFA including culture of a microorganism capable of producing arachidonic acid (ARA) and / or dihomo-gamma-linolenic acid (DGLA) is provided, the method including at least one of the following steps: (a) adding an organic acid in an amount of 0.01 to 5 w / v % to a culture medium after the beginning of main culture; (b) increasing the pH of the culture medium to a range effective for culture after the beginning of the main culture; and (c) adding a metal sulfate in an amount of 0.01 to 0.5 w / w % to the main culture medium.
Owner:SUNTORY HLDG LTD
Plasma flying ash melting tail gas wet-purification process
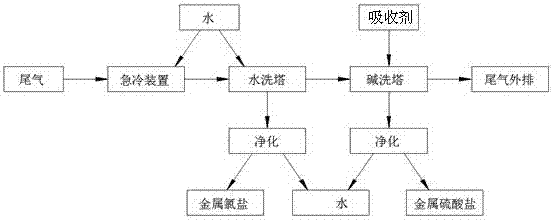
InactiveCN107008127AImprove heat transfer efficiencyImprove mass transfer efficiencyDispersed particle separationLiquid wasteQuenching
The invention relates to a plasma flying ash melting tail gas wet-purification process. The process includes: after plasma flying ash melting tail gas is cooled to 100 DEG C or below by a quenching device, sequentially feeding into a water washing tower and an alkaline washing tower to realize deacidification, discharging smoke to the atmosphere from the alkaline washing tower, respectively purifying waste liquid discharged from the water washing tower and the alkaline washing tower, and recycling obtained metal chlorine salts and metal sulfuric acid salts. The purification process has advantages of completeness and effectiveness in removal of acid gases and the metal chlorine salts, heavy metal salt enrichment is avoided, HCl and SO2 in the tail gas are separated from the source of the tail gas purification process, and the two metal salts are recycled through a water treatment device, so that pollutants in the tail gas are effectively recycled.
Owner:JIANGSU TIANYING ENVIRONMENTAL PROTECTION ENERGY COMPLETE EQUIP CO LTD
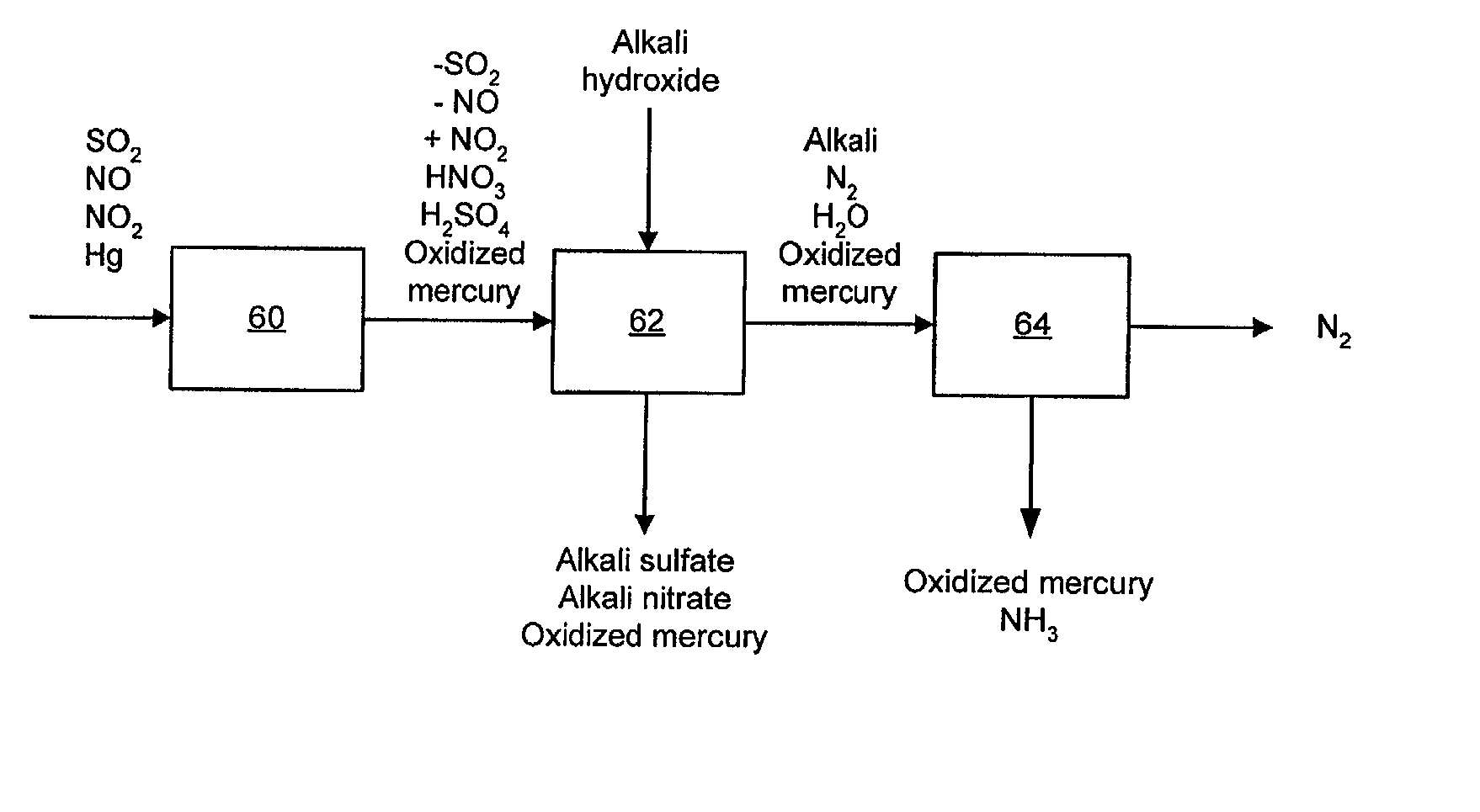
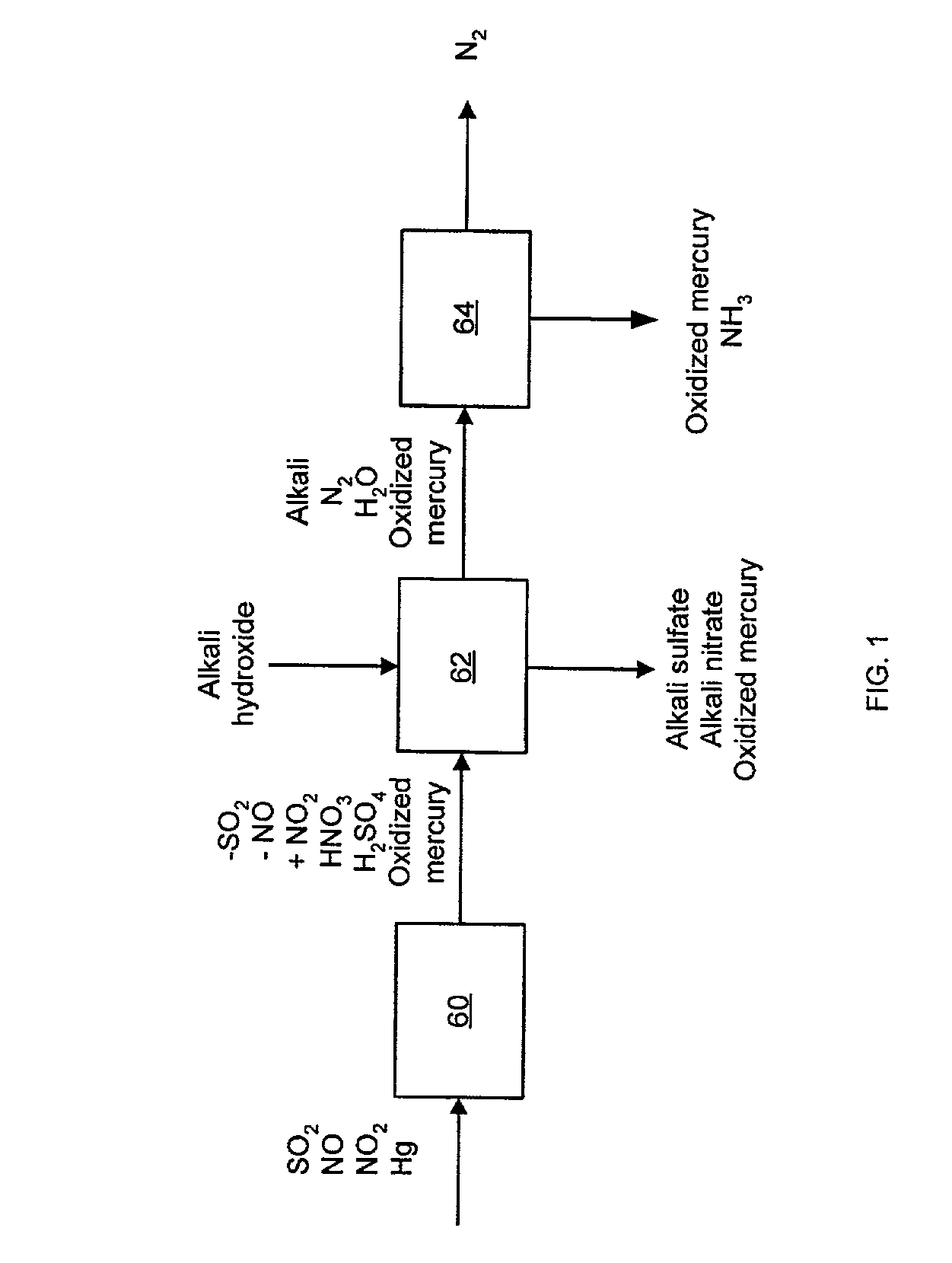
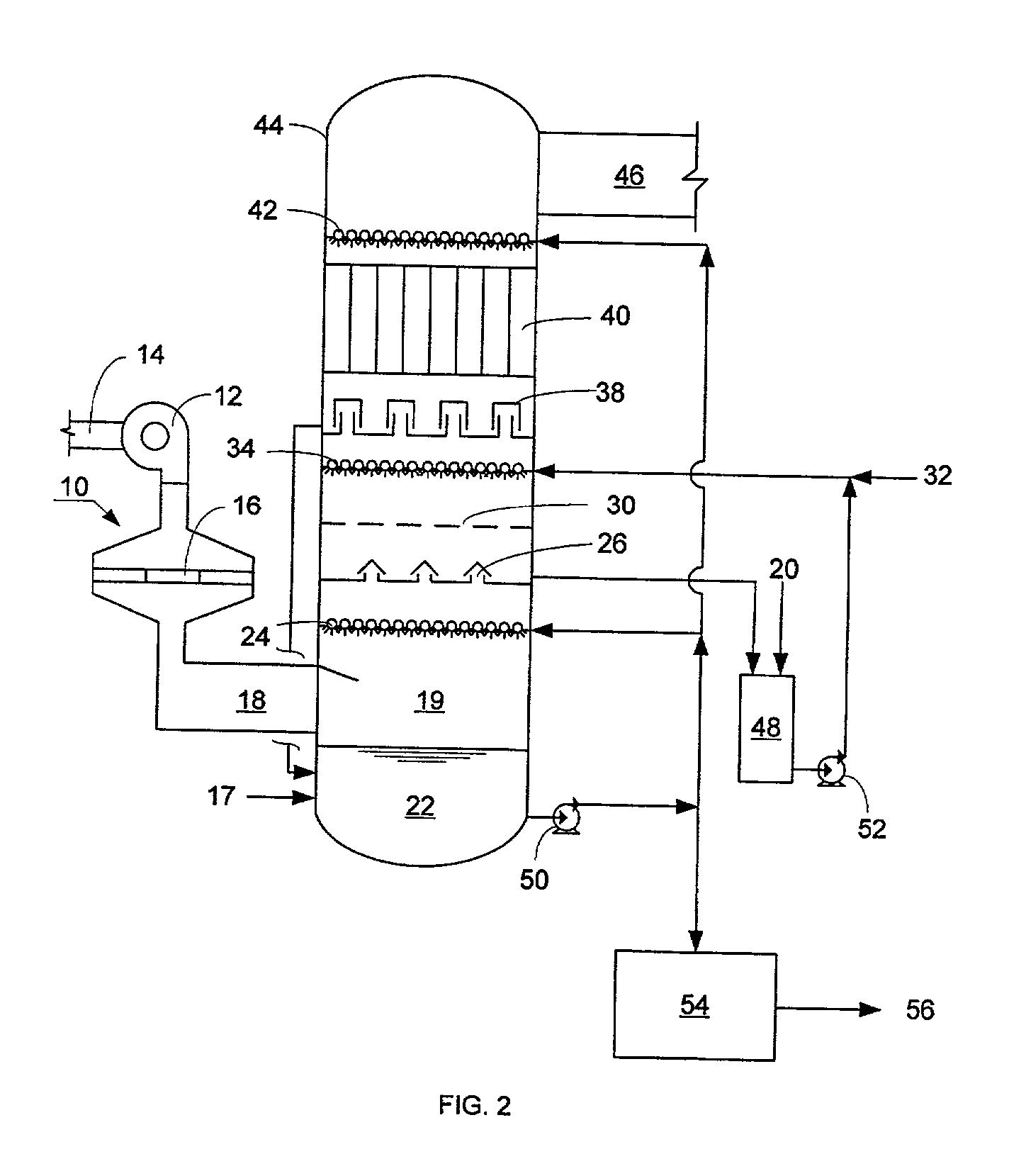
NOx, Hg, and SO2 removal using alkali hydroxide
A process and apparatus for removing SO2, NO, and NO2 from a gas stream having the steps of oxidizing a portion of the NO in the flue gas stream to NO2, scrubbing the SO2, NO, and NO2 with an alkali scrubbing solution, and removing any alkali aerosols generated by the scrubbing in a wet electrostatic precipitator. The process can also remove Hg by oxidizing it to oxidized mercury and removing it in the scrubbing solution and wet electrostatic precipitator. Alkali sulfates, which are valuable fertilizers, can be withdrawn from the rubbing solution.
Owner:POWERSPAN CORP
Method for preparing decoloring material of dyeing waste water by attapulgite ore
InactiveCN1843950AWith physical adsorptionExcellent chemical flocculation and decolorizationWater/sewage treatment by flocculation/precipitationWater/sewage treatment by sorptionManganeseRoom temperature
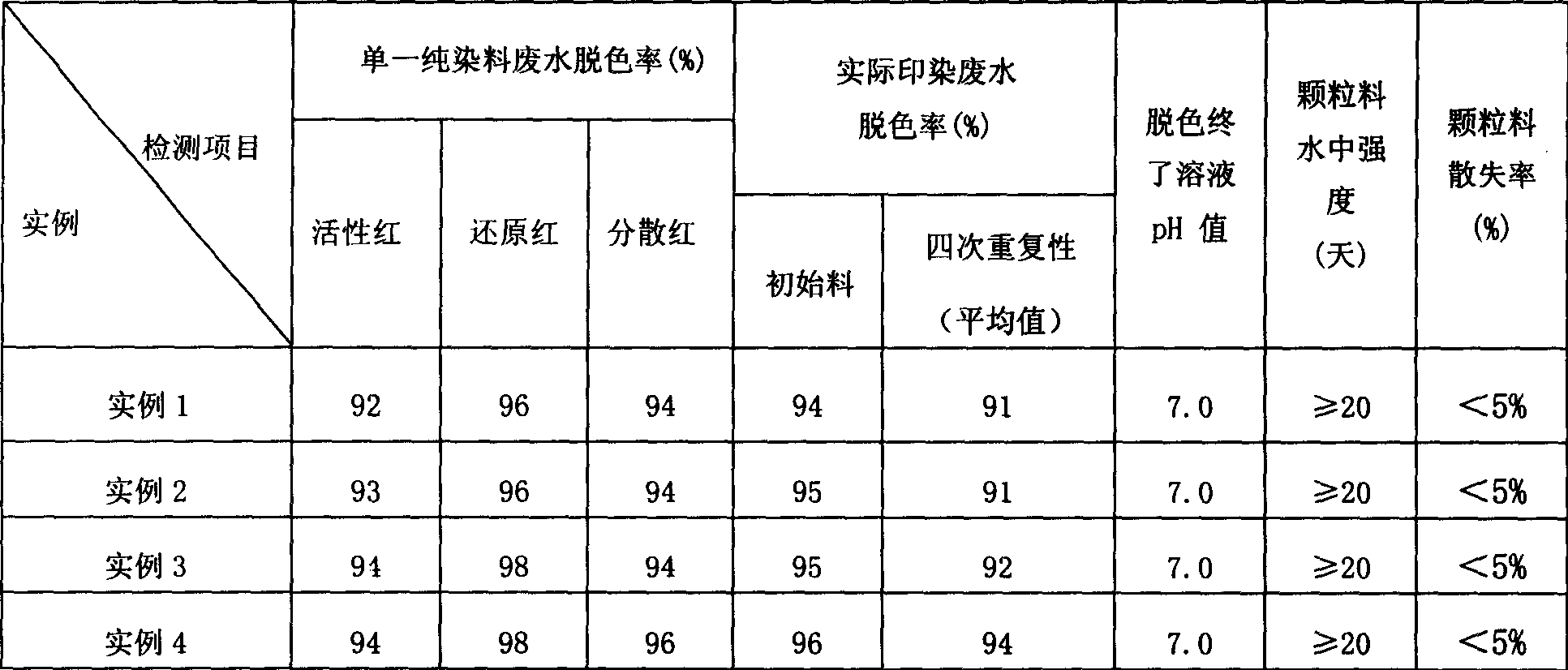
The invention relates to method for preparing decolouring material for printing and dyeing wastewater, which employs concave-convex stick mineral as main raw material (80-100 mu), mixing with sulfuric acid of 1.5-3.0 mol / l, the ratio between solid and liquid is 1:2-3, activating under room temperature for 1-2 hours; adding 0-5% of metallic sulphate such as iron, manganese and alumium (weight by metallic oxide); neutralizing with basic solution of 3-6 mol / l to make pH be about 7-8; solid-liquid separating, granulating (3-5 mm), drying, calcining under 700 Deg. C for 0.5-2 hours, and getting product. The invention can get side product of Na2SO4 10H2O or (NH4)2SO4 from filtering liquor; the decoloring material can be reused for more than four times after being immersed with ammonia sulfate of 1.5-6.0 mol / l for 2-5 minutes and calcined under 300 Deg.C for 5-25 minutes; the decoloration rate is over 94%, and decoloration rate with reused decoloraing material is over 91%. The invention is characterized by simple producing process, low cost and no pollution.
Owner:SICHUAN UNIV
Oil-stain cleaning agent as well as preparation method and use method thereof
ActiveCN104342327AEfficient removalEffective absorptionDetergent mixture composition preparationSurface-active non-soap compounds and soap mixture detergentsMohs scale of mineral hardnessHardness
The invention provides an oil-stain cleaning agent. The oil-stain cleaning agent comprises a main cleaning material and an auxiliary cleaning material, wherein the main cleaning material comprises microcrystal powder particles with crystalline structures and mohs hardness of 2-4; the surfaces of the microcrystal powder particles are wrapped and coated with a layer of water-proof material; the microcrystal powder particles comprise the following components: metal sulfate and metal carbonate; and the auxiliary cleaning material comprises a nonionic surface active agent, a corrosion inhibiting agent and an anti-rusting agent. The invention also provides a preparation method and a use method of the oil-stain cleaning agent.
Owner:厦门泰益新洁净科技有限公司
Process for treating acid mine water with moderate to high sulfate content
InactiveUS6790352B1Waste water treatment from quariesSolid sorbent liquid separationAcid mine drainageAmmonia
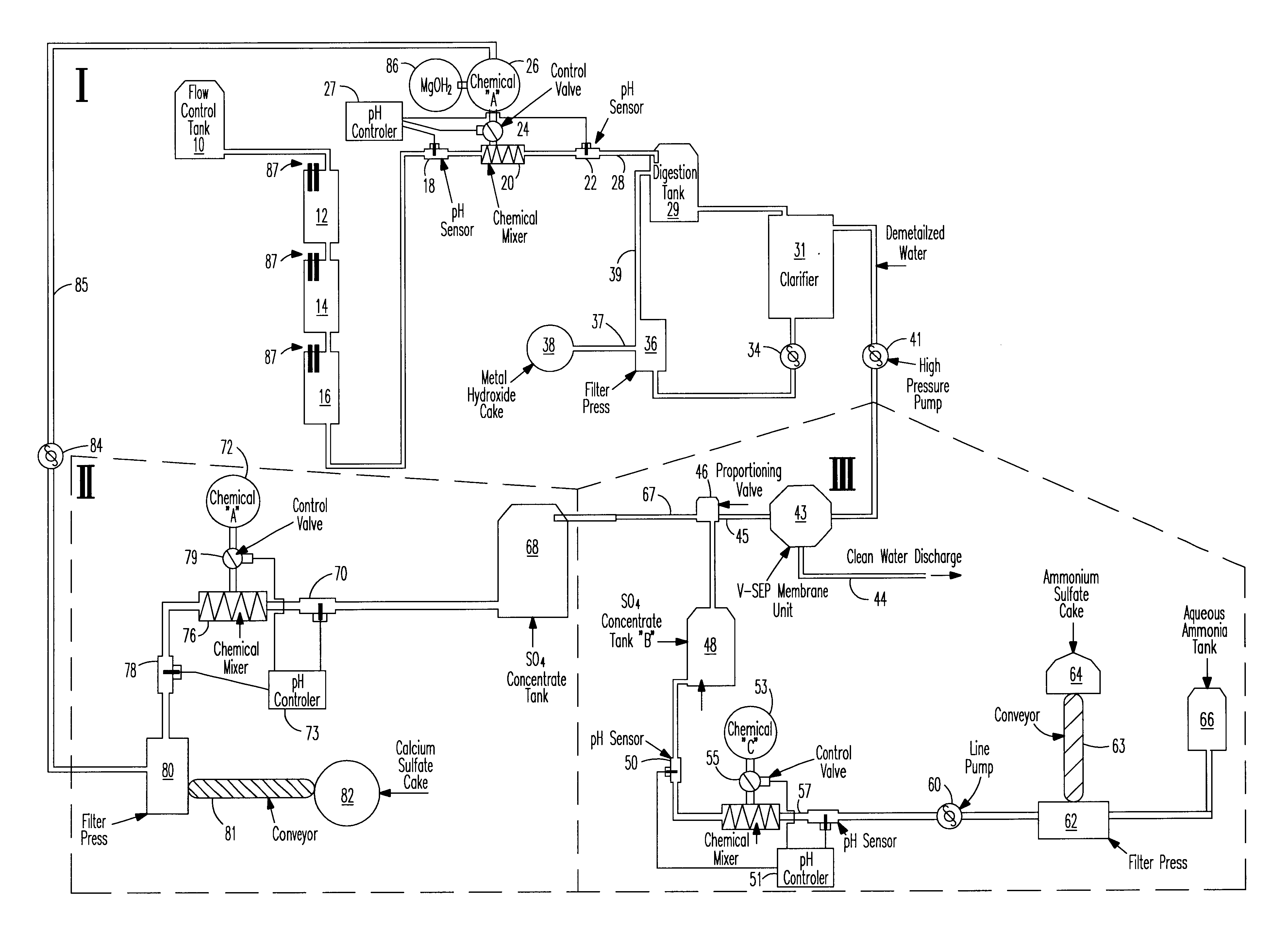
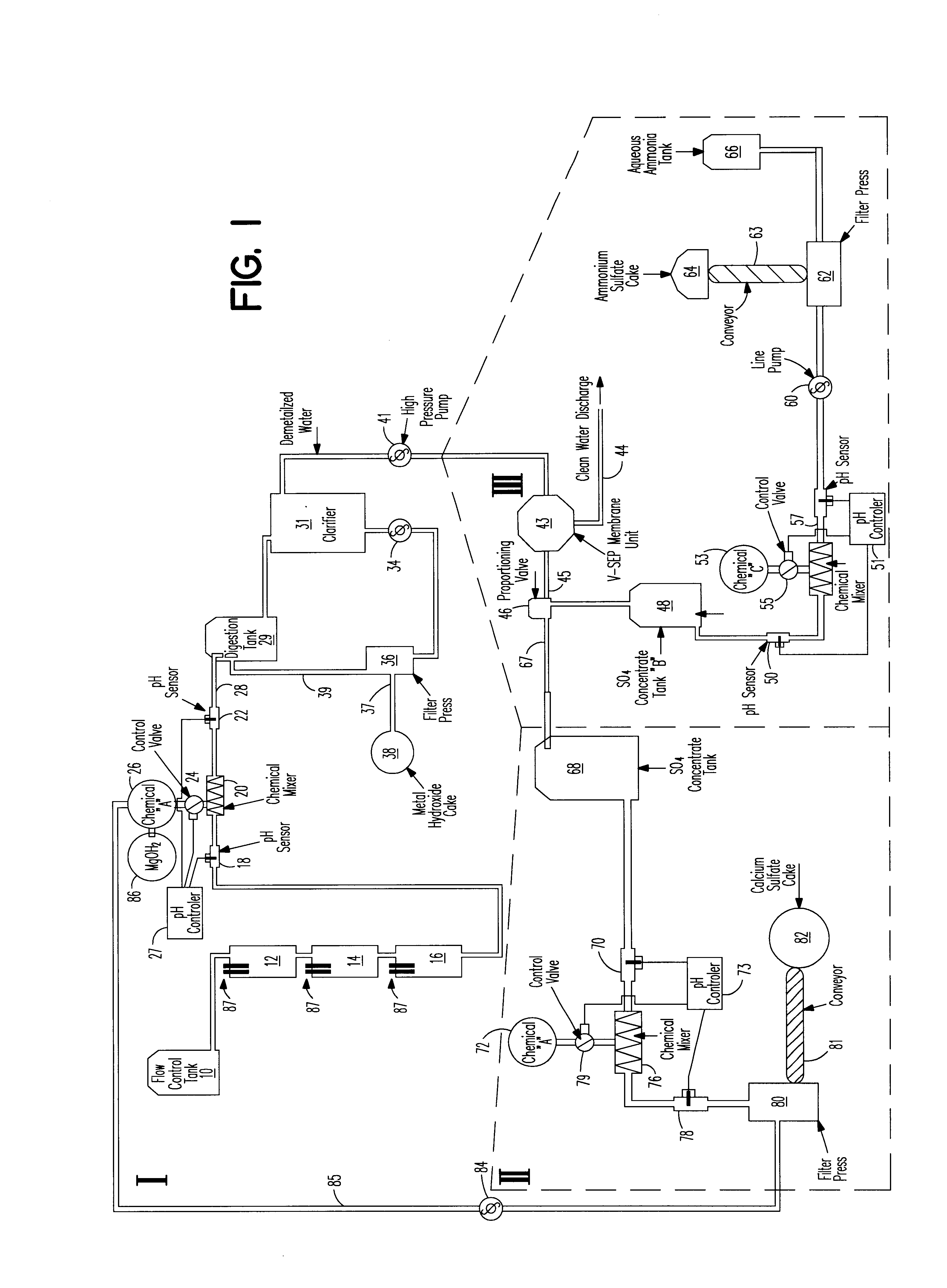
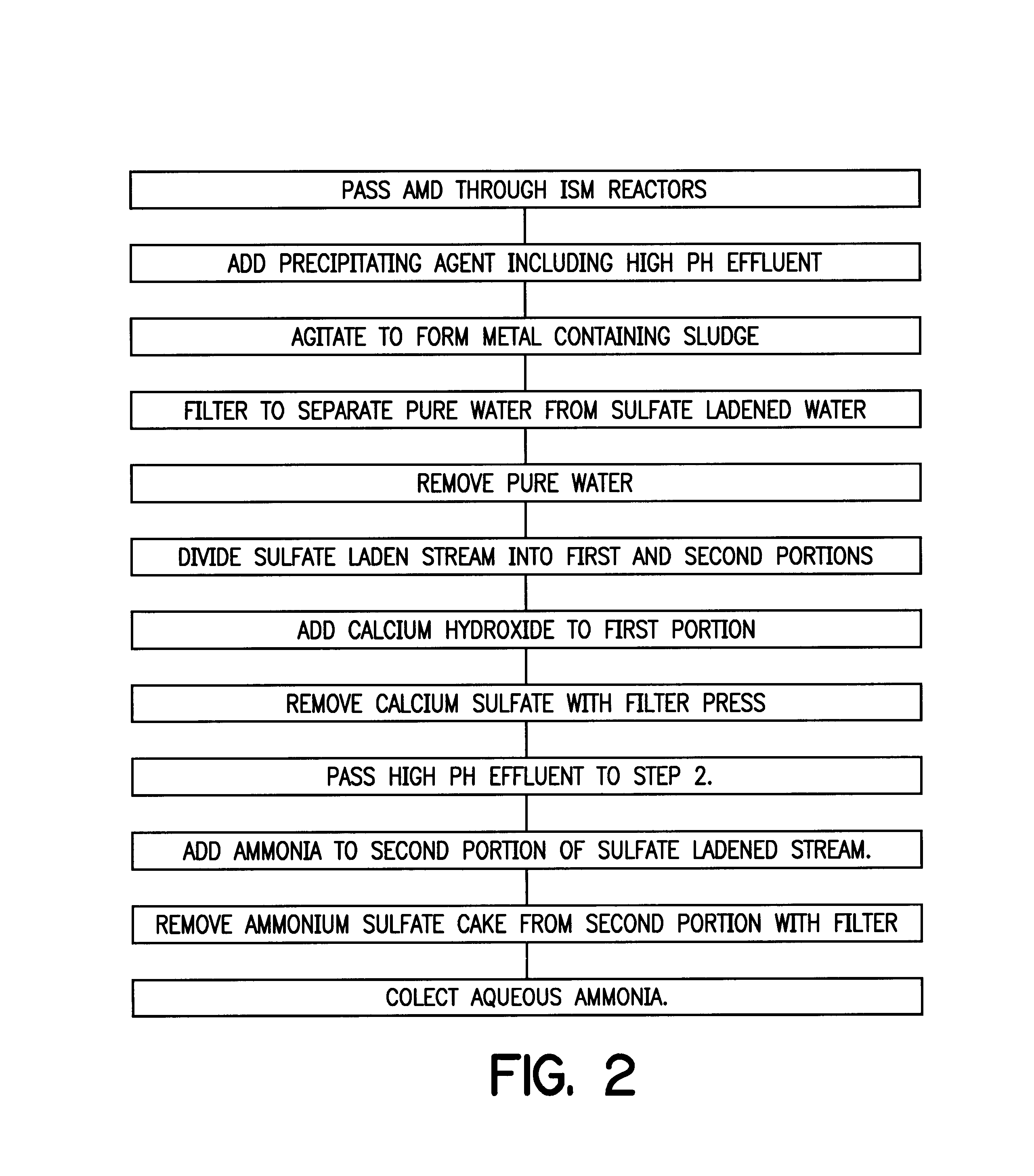
An apparatus for treating water contaminated with metal sulfates and sulfuric acid such as acid mine drainage (AMD) which in part recycles high pH effluent from later steps in the process back to the earlier steps of the process. The recycled high pH effluent added with magnesium hydroxide to the entering AMD generates precipitates separable from the stream to leave sulfate ladened water. A tangential filtering process is used to separate the sulfate ladened water into one stream of pure water and a second stream containing sulfate. One portion of the second stream is treated with ammonia to yield a cake of ammonia sulfate and aqueous ammonia. Ca(OH)2 is added to another portion of the second stream to produce calcium sulfate cake and the high pH effluent that is recycled back to the first step in the cycle.
Owner:MILLSTONE PROPERTIES
Coagulants made in situ from sulfate-containing water and uses therewith
InactiveUS20100187130A1Promote flocculationFacilitates oxidationWaste water treatment from quariesWater contaminantsSulfate radicalsSoil science
Acid mine drainage and surface waters containing sulfate are processed by an electrocoagulator to make aluminum or other polyvalent metal sulfate, which acts as a coagulant for solids suspended in the waters. The process thus removes and puts to good use highly undesirable sulfate anions, obviating combinations with barium and other scale forming metals when the fluids are used in well drilling for other purposes associated with hydrocarbon recovery. Well fluids may be treated with the acid mine drainage including the sulfate coagulant made in it. Efficiency of the process may be enhanced by the addition of an oxidizing agent and / or by passing the fluid through a cavitation device or other mechanism to improve mixing, enabling the process to handle large quantities of acid mine drainage and fluids handled in hydrocarbon recovery, particularly from shale formations.
Owner:TOTAL SEPARATION SOLUTIONS
Process to produce simonkolleite, zinc oxide and zinc hydroxide
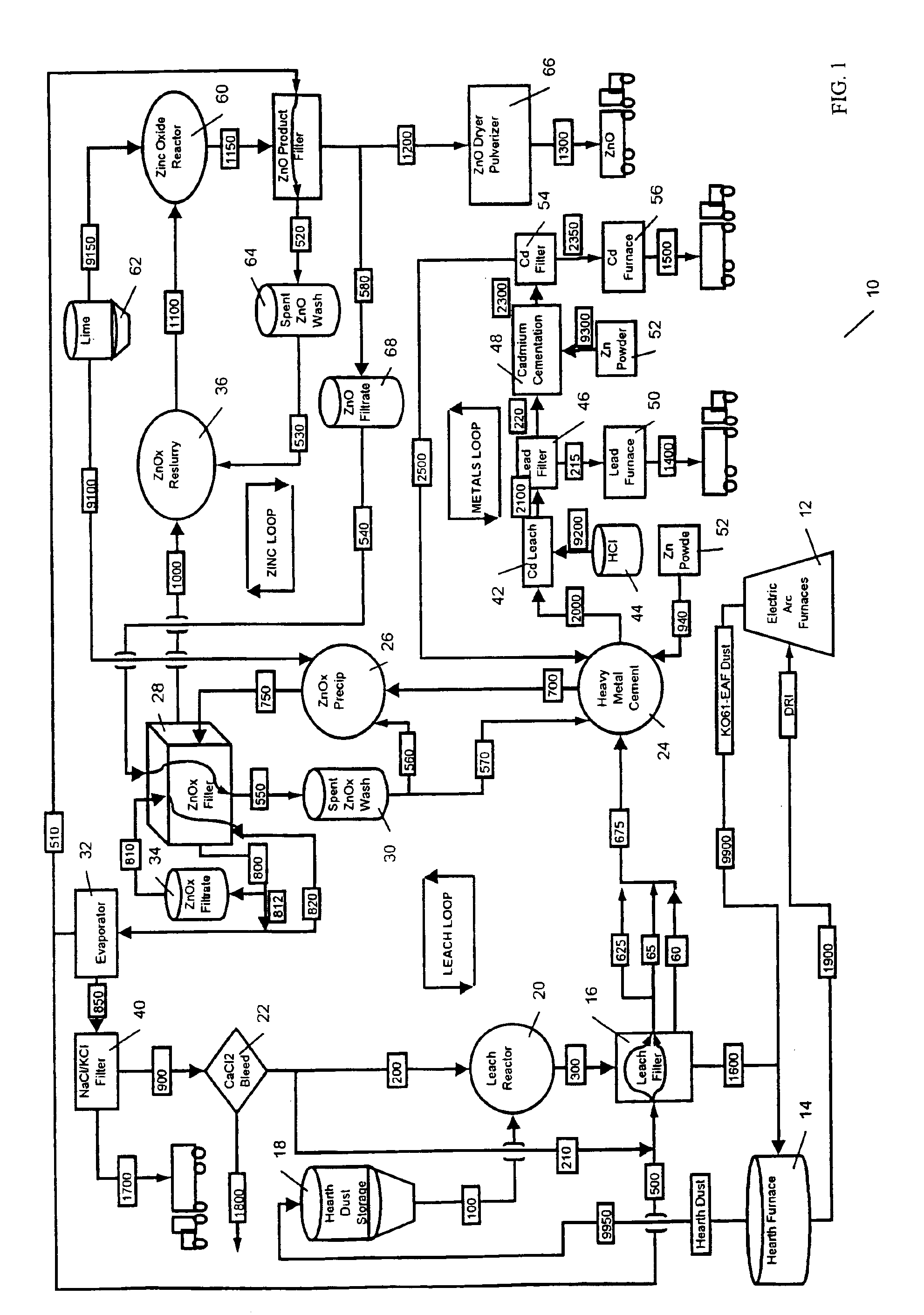
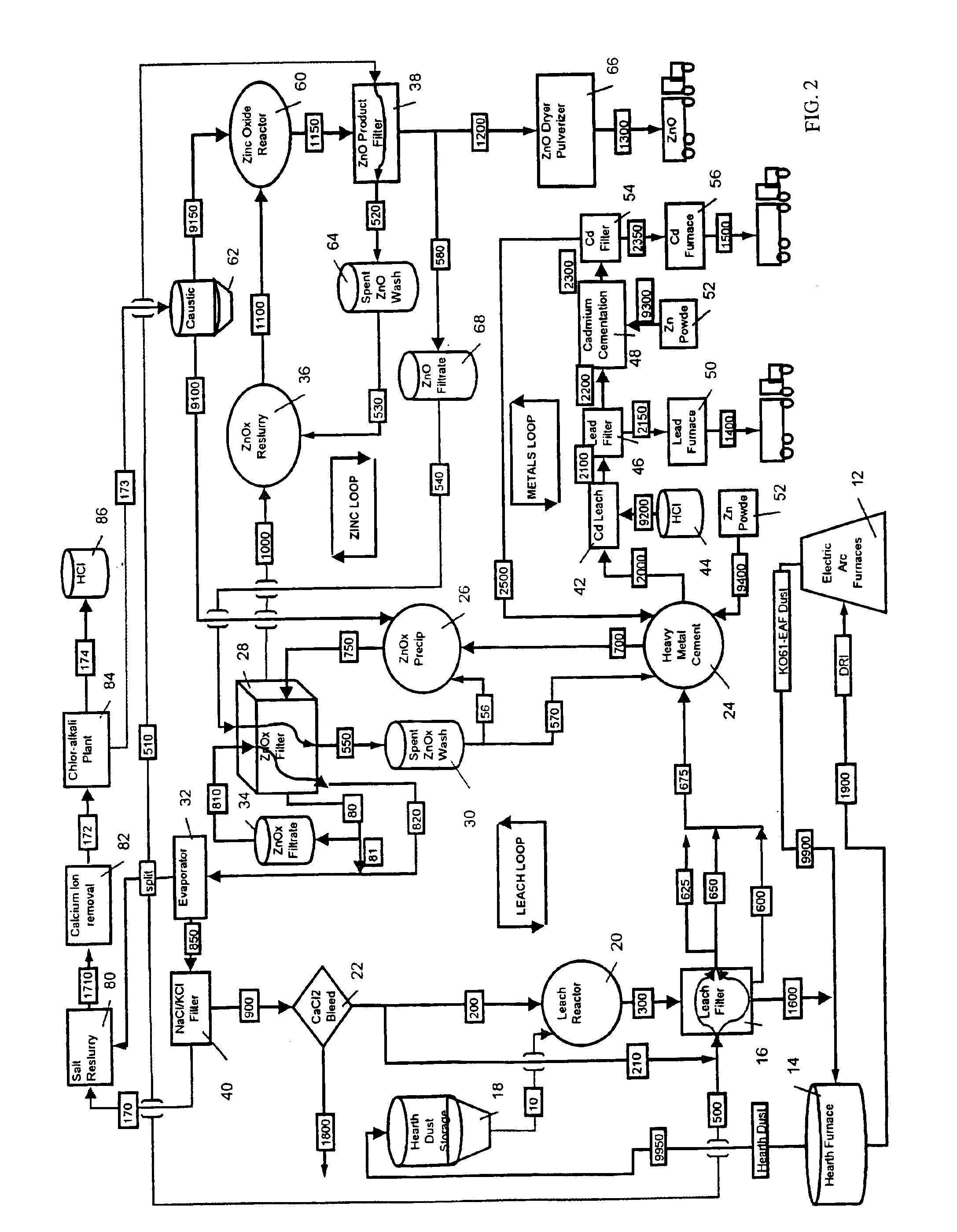
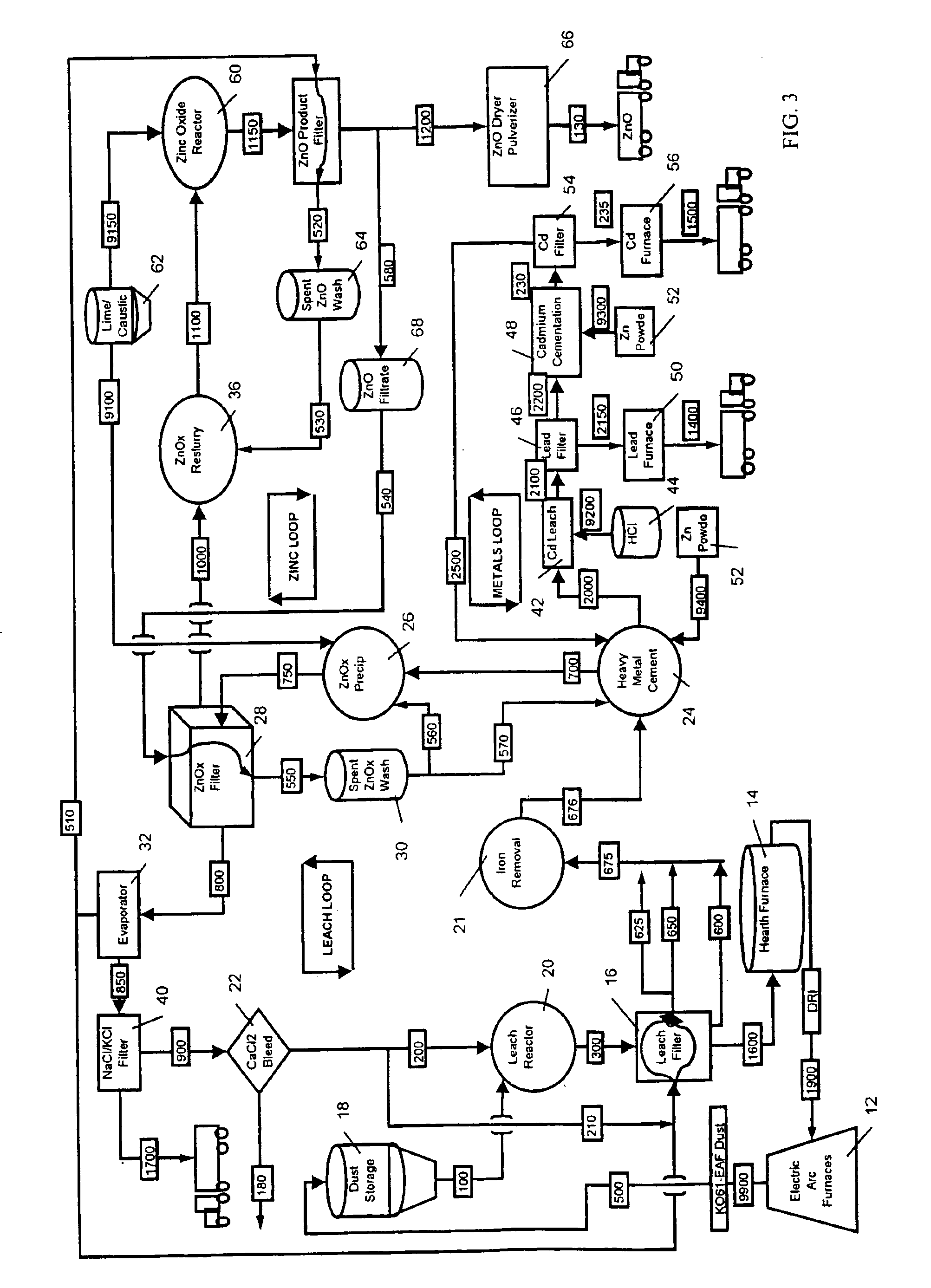
A hydrometallurgical process utilizing an atmospheric calcium chloride leach to selectively recover from various metal feed stocks (consisting of elemental metals, metal oxides, metal ferrite, metal hydroxide, metal carbonates, metal sulfate / sulfur compounds, and their hydrates, specifically including but not limited to EAF Dust K061) zinc, lead, cadmium, silver, copper and other valuable metals to the exclusion of iron, magnesium, halogen salts and other unwanted elements. The process solves the problem of iron and magnesium leach solution contamination because iron is unexpectedly converted to magnetite. The heavy metals are cemented out of solution using zinc or other selected dust at a pH of 6 or greater under unique and unexpected conditions, which do not require acid. Simonkolleite / zinc- oxychloride / zinc-hydroxide is produced from the purified zinc chloride complex pregnant leach solution and is converted directly to high purity active rubber grade 99+% zinc oxide having small particle size and high surface area. The products are metal concentrates suitable for: metal refiner / processors, production of elemental metal, or other conversion processes. The process removes Arsenic and Fluorides in the feed material. The process also solves the problem of chloride contamination in the zinc oxide and prevents heavy metal contaminants in the hydrometallurgically produced zinc oxide derived from feed stocks containing chlorides or when chlorides are used to leach the metal bearing feed stocks. In one embodiment, calcium and / or magnesium compounds are added to the iron bearing waste to increase the recovery of zinc and other non-ferrous metals and to produce an iron bearing flux. The process is environmentally friendly and fully recycles all streams.
Owner:WHITMAN CHESTER W
Metal ore heap leaching, anaerobic enrichment transformation and biological leaching extraction process
InactiveCN102534210ALow concentration requirementEliminate pollutionProcess efficiency improvementLiquid wasteHigh concentration
The invention relates to a metal ore heap leaching, anaerobic enrichment transformation and biological leaching extraction process, which comprises the following steps: utilizing metal sulphate solution with low concentration obtained in the open-air heap leaching operation and treating through a sulfate reduction bacteria process (anaerobic bioreactor) to obtain a metal sulfide precipitate (solid); and obtaining metal sulphate solution with high concentration through bio-leaching (bio-leaching reactor). The invention has the beneficial effects that the environmental risk of external leakage of leaching solution is reduced, the heap leaching period is shortened, the storage capacity of a heap leaching field is greatly reduced, the environmental risk caused by accidental leakage is reduced, and the concentration of metal ions in treated waste liquid is kept below dozens of micrograms per liter so as to be lower than the current environment protection standard requirements.
Owner:JIANGXI UNIV OF SCI & TECH
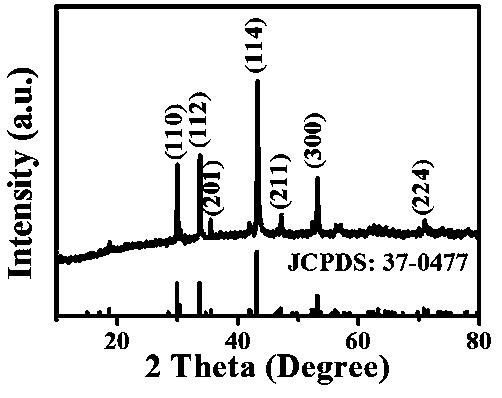
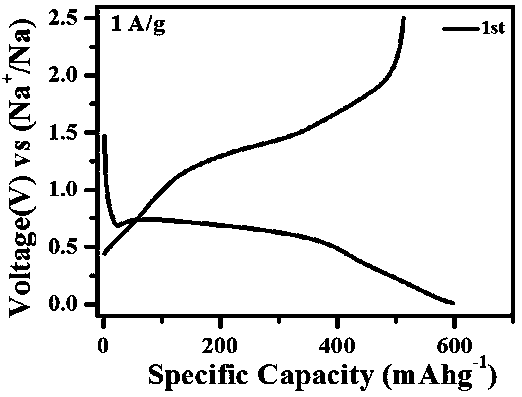
Carbon-coated ferrous sulfide negative electrode material, preparation method and sodium ion battery prepared by the same
ActiveCN109167035AEasy to makeAbundant raw materialsCell electrodesSecondary cellsCarbon coatingElectrical battery
The invention relates to a carbon-coated ferrous sulfide negative electrode material, the preparation method and the prepared sodium ion battery thereof, Carbon-coated ferrous sulfide anode material refers to carbon-coated ferrous sulfide and its doped modified material, wherein the particle size of the ferrous sulfide is 100-500 nm, the mass of the carbon coating layer is 3-30% of the mass of theelectrode material, the doping modification elements are one or several of M=Co, Ni, Mn, Ti, Cu, Mg, Ba, Pb, Al, etc., and the mass of the doping modification elements is 0-30% of the mass of the negative electrode material. A metal sulfate and a carbon source are added into deionized water, stirred and dissolved uniformly at room temperature, freeze-dried to obtain a precursor, and calcined at high temperature in an inert atmosphere to obtain a carbon-coated ferrous sulfide negative electrode material. As that negative electrode material of the sodium ion battery, the invention has the advantage of abundant raw materials, simple preparation, short preparation period, low cost, no pollution, good rate performance and strong cycle stability, and is suitable for large-scale production.
Owner:ZHENGZHOU UNIV
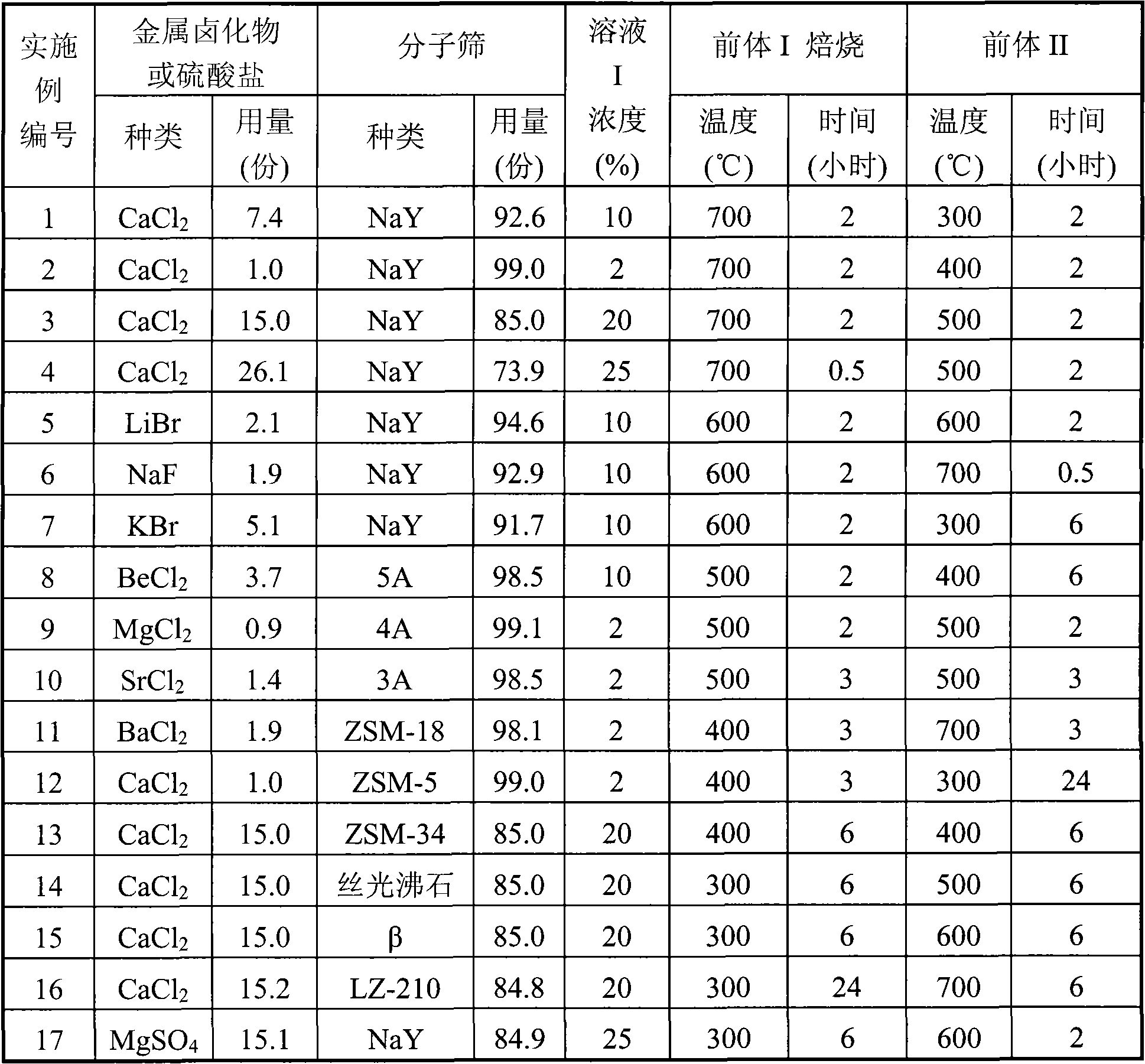
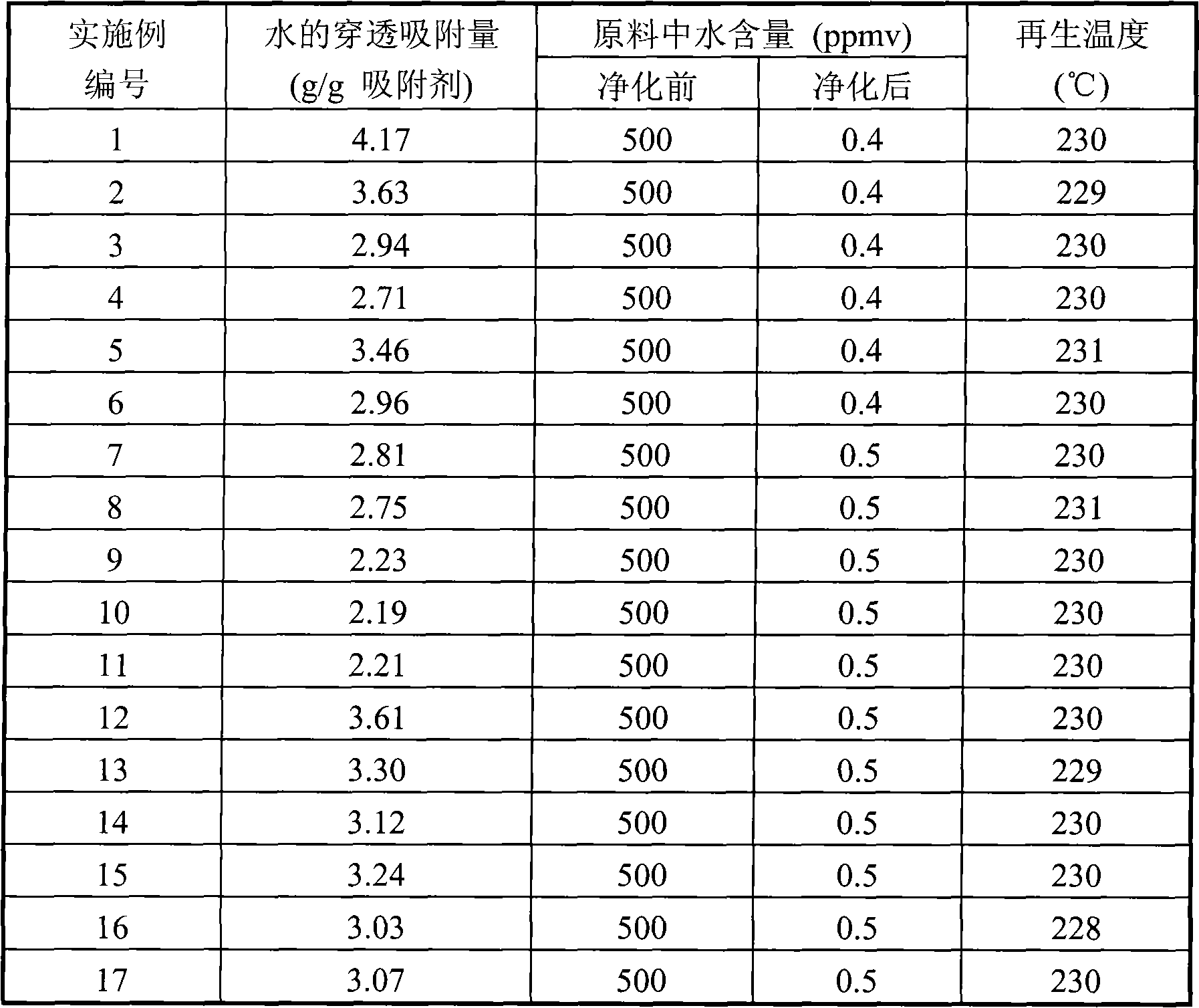
Solid adsorbent and preparation method thereof
ActiveCN101884905AHigh adsorption capacityImprove adsorption capacityOther chemical processesMolecular sieveAlkaline earth metal
The invention relates to a solid adsorbent and a preparation method thereof, mainly solving the problems of low adsorption capacity and higher regeneration temperature of the adsorbent in the prior art. By adopting the technical scheme that a compound solid adsorbent is prepared by combining at least one kind of halide of alkali metal, halide of alkaline earth metal or metal sulfates with a molecular sieve, the invention better solves the problems and can be used for industrial production of adsorption and purification of various hydrocarbon raw materials.
Owner:CHINA PETROLEUM & CHEM CORP +1
Magnesium base flue gas wet desulfurization catalyst
InactiveCN103949287AHigh activityIncrease concentrationOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationOrganic acidFlue gas
The present invention relates to a magnesium base flue gas wet desulfurization catalyst, which can be poured into a desulfurization absorption tower so as to prompt a desulfurization medium magnesium base compound well be dispersed in a desulfurization slurry, and contains an organic acid, an organic acid sodium salt and a metal sulfate. According to the present invention, the catalyst or synergist is added to the desulfurization absorption tower, such that the activity, the concentration and the mass transfer of the desulfurization element magnesium ions can be increased, and the oxidation process of magnesium sulfite can be accelerated so as to increase the sulfur dioxide removal rate and achieve the purposes of energy saving and emission reducing.
Owner:党晓军
Resin composition, multilayer structure using same and method for producing same
ActiveUS20120128961A1ViscosityHigh viscositySynthetic resin layered productsDomestic containersVinyl esterViscosity
Disclosed is a resin composition comprising (A) a saponified ethylene-vinyl ester copolymer and (B) a completely or partially dehydrated polyvalent metal sulfate hydrate. Preferably, the component (B) is a completely or partially dehydrated 2-valent metal sulfate hydrate. The resin composition can suppress increasing viscosity in preparation thereof as well as production of the multilayer structure therefrom. A molded article can be stably produced and recover quickly gas barrier property deteriorated by hot water treatment and additionally maintain the superior gas barrier property.
Owner:MITSUBISHI CHEM CORP
Process for the preparation of nitrobenzene by adiabatic nitration
ActiveUS7781624B2Low boiling pointLower energy requirementsNitro compound preparationBenzeneNitrobenzene
This invention relates to a process for the continuous preparation of nitrobenzene. This process comprises the adiabatic nitration of benzene with a mixture of sulfuric acid and nitric acid, in which the sum of the concentrations in the reaction zone of the metal ions which form sparingly soluble metal sulfates is less than 900 mg / l, based on the volume of the aqueous phase which contains sulfuric acid.
Owner:COVESTRO DEUTSCHLAND AG
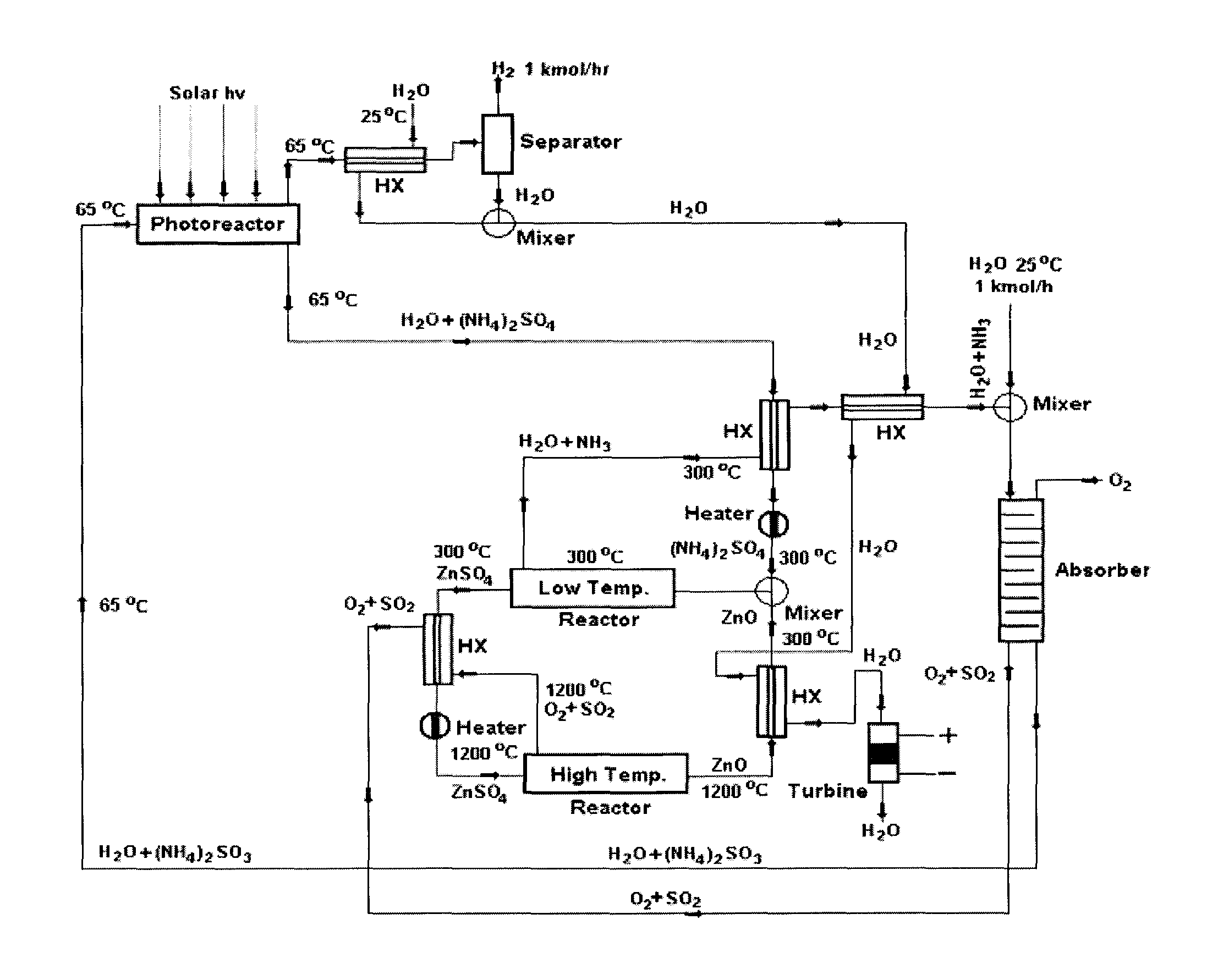
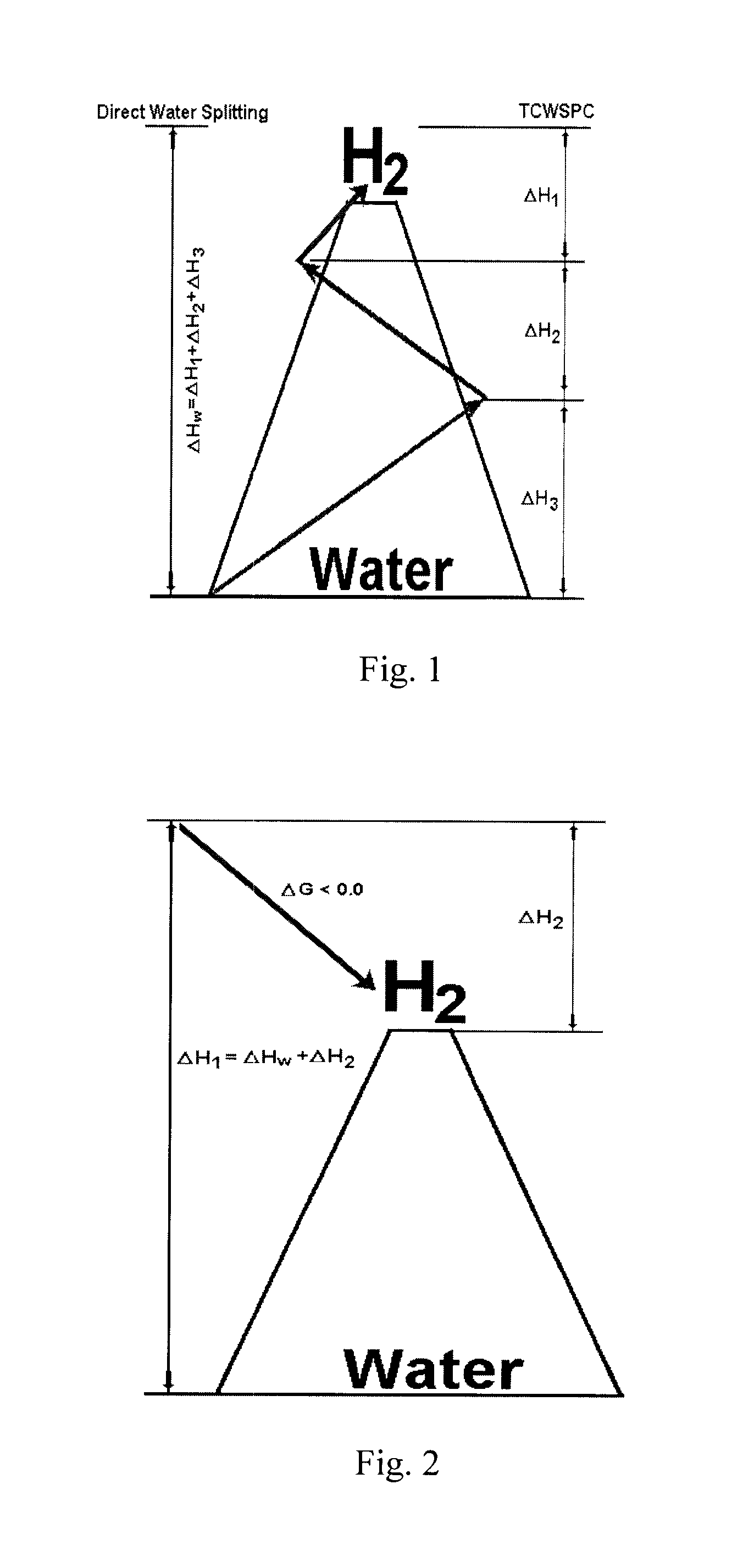
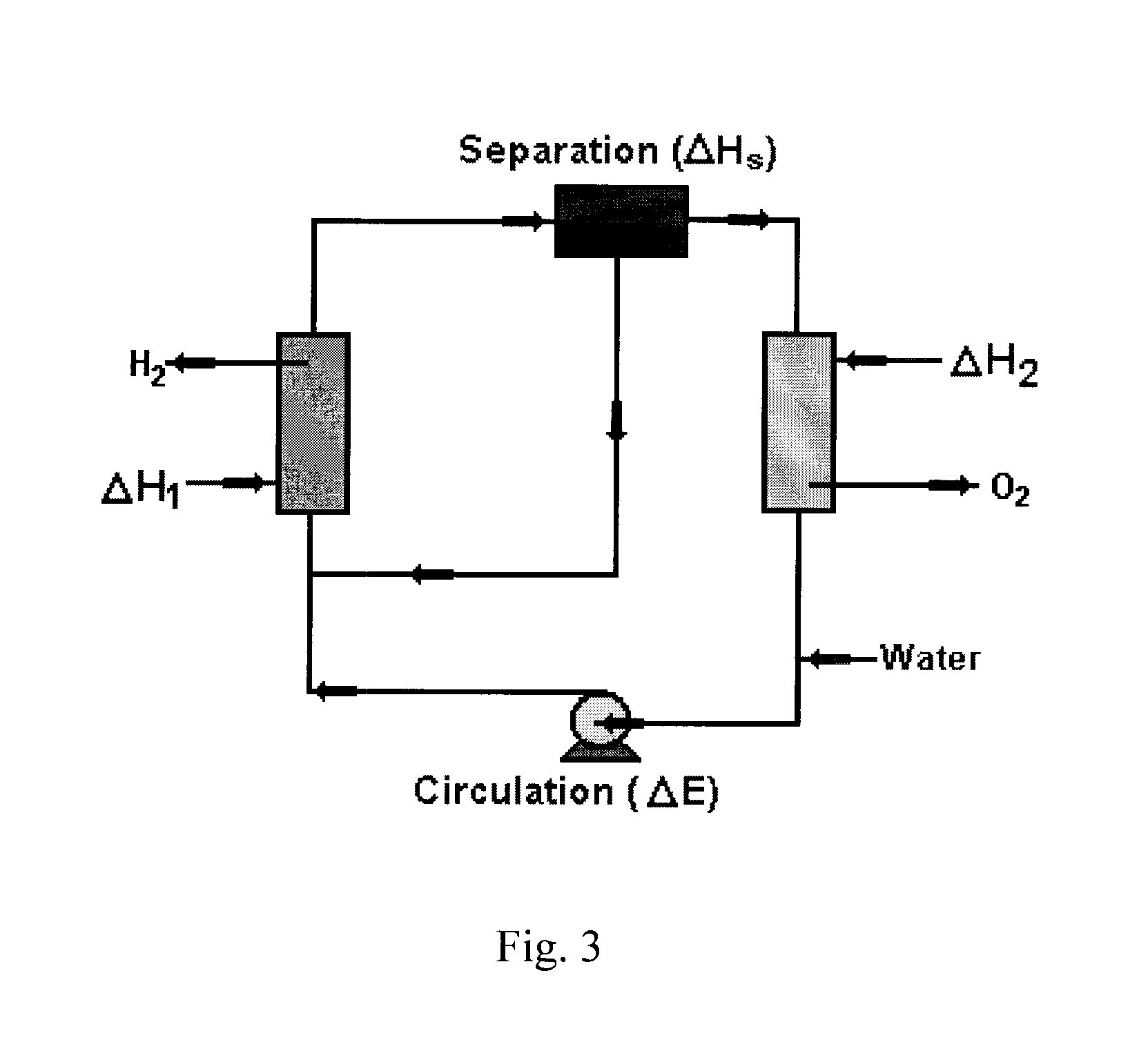
Solar metal sulfate-ammonia based thermochemical water splitting cycle for hydrogen production
InactiveUS8691068B1Improve efficiencyEasy to separateOxygen/ozone/oxide/hydroxideElectrolysis componentsPhotocatalytic reactionSlurry
Two classes of hybrid / thermochemical water splitting processes for the production of hydrogen and oxygen have been proposed based on (1) metal sulfate-ammonia cycles (2) metal pyrosulfate-ammonia cycles. Methods and systems for a metal sulfate MSO4—NH3 cycle for producing H2 and O2 from a closed system including feeding an aqueous (NH3)4SO3 solution into a photoctalytic reactor to oxidize the aqueous (NH3)4SO3 into aqueous (NH3)2SO4 and reduce water to hydrogen, mixing the resulting aqueous (NH3)2SO4 with metal oxide (e.g. ZnO) to form a slurry, heating the slurry of aqueous (NH4)2SO4 and ZnO(s) in the low temperature reactor to produce a gaseous mixture of NH3 and H2O and solid ZnSO4(s), heating solid ZnSO4 at a high temperature reactor to produce a gaseous mixture of SO2 and O2 and solid product ZnO, mixing the gaseous mixture of SO2 and O2 with an NH3 and H2O stream in an absorber to form aqueous (NH4)2SO3 solution and separate O2 for aqueous solution, recycling the resultant solution back to the photoreactor and sending ZnO to mix with aqueous (NH4)2SO4 solution to close the water splitting cycle wherein gaseous H2 and O2 are the only products output from the closed ZnSO4—NH3 cycle.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
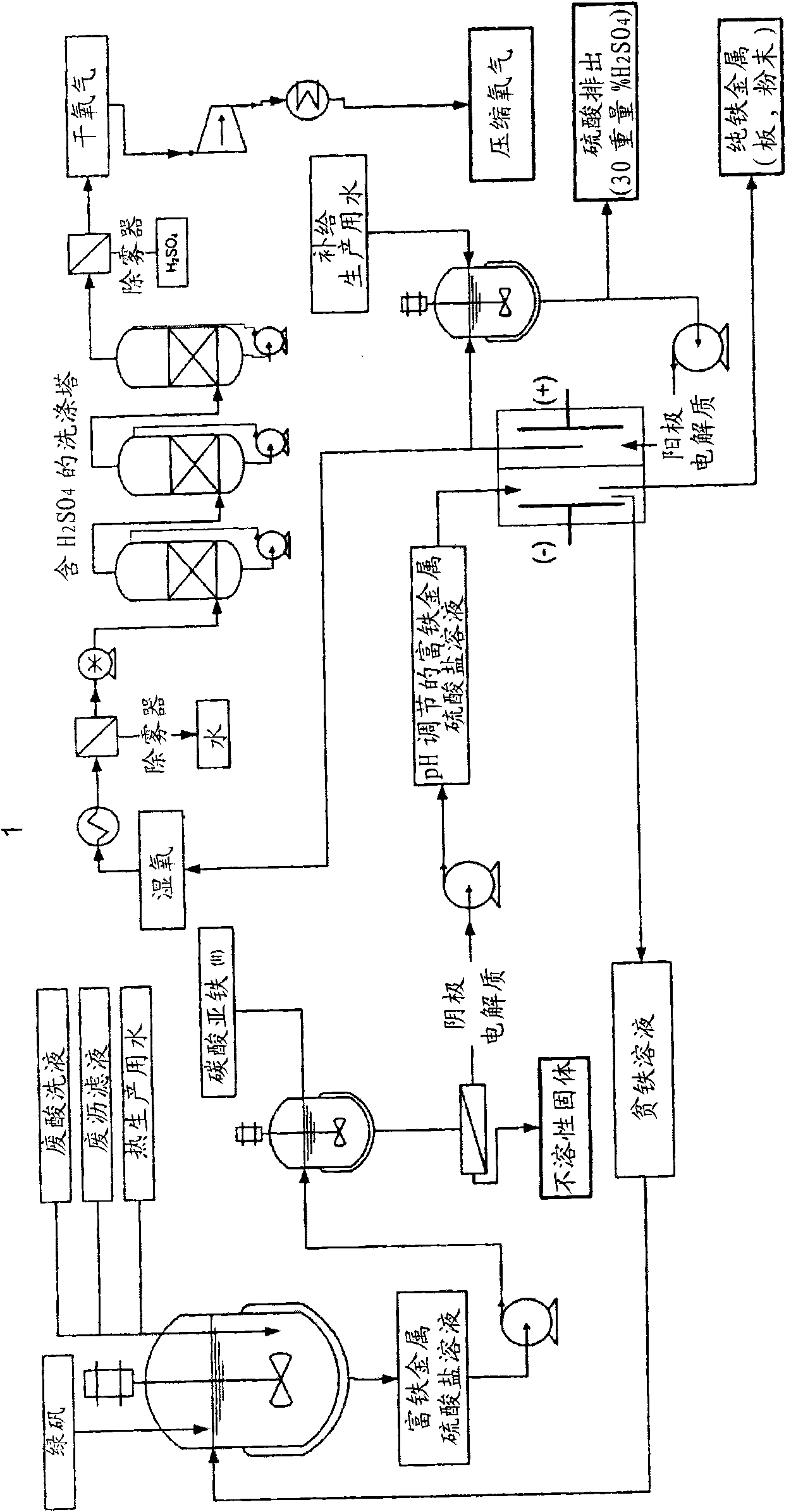
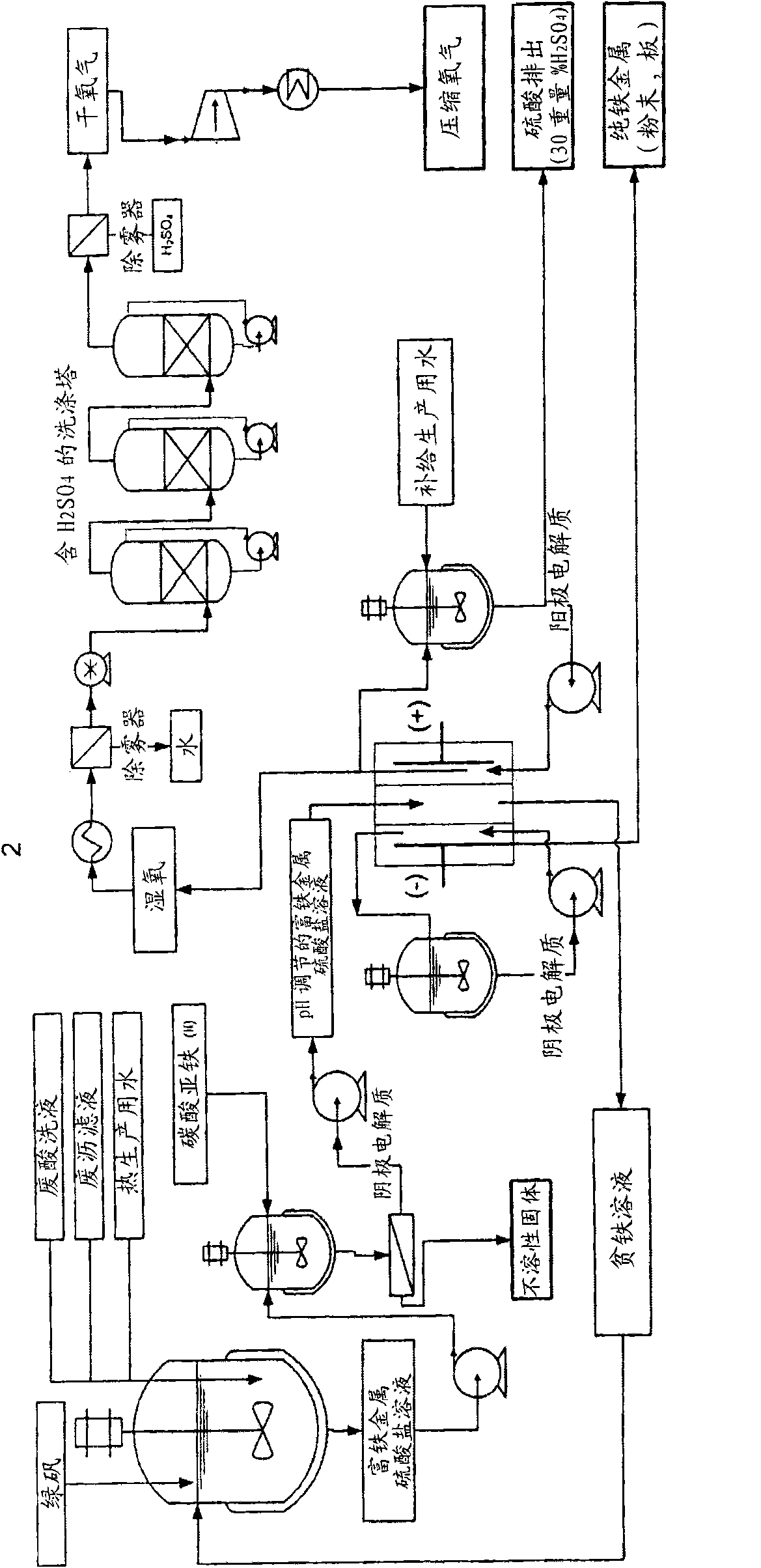
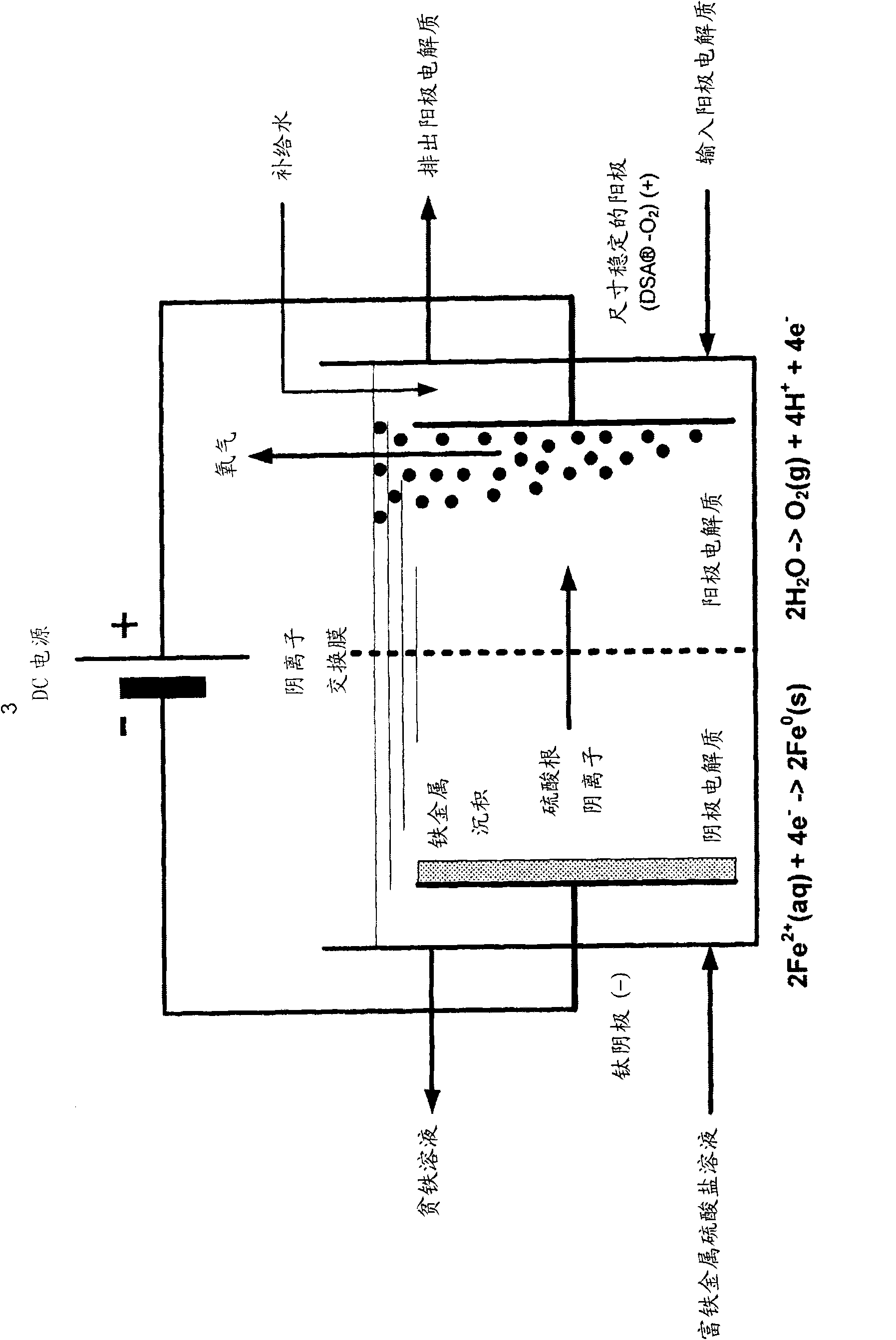
Electrochemical process for the recovery of metallic iron and sulfuric acid values from iron-rich sulfate wastes, mining residues and pickling liquors
An electrochemical process for the recovery of metallic iron or an iron-rich alloy, oxygen and sulfuric acid from iron-rich metal sulfate wastes is described. Broadly, the electrochemical process comprises providing an iron-rich metal sulfate solution; electrolyzing the iron-rich metal sulfate solution in an electrolyzer comprising a cathodic compartment equipped with a cathode having a hydrogen over- potential equal or higher than that of iron and containing a catholyte having a pH below about 6.0; an anodic compartment equipped with an anode and containing an anolyte; and a separator allowing for anion passage; and recovering electrodeposited iron or iron-rich alloy, sulfuric acid and oxygen gas. Electrolyzing the iron-rich metal sulfate solution causes iron or an iron-rich alloy to be electrodeposited at the cathode, nascent oxygen gas to evolve at the anode, sulfuric acid to accumulate in the anodic compartment and an iron depleted solution to be produced.
Owner:弗朗索瓦·卡达雷利
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com