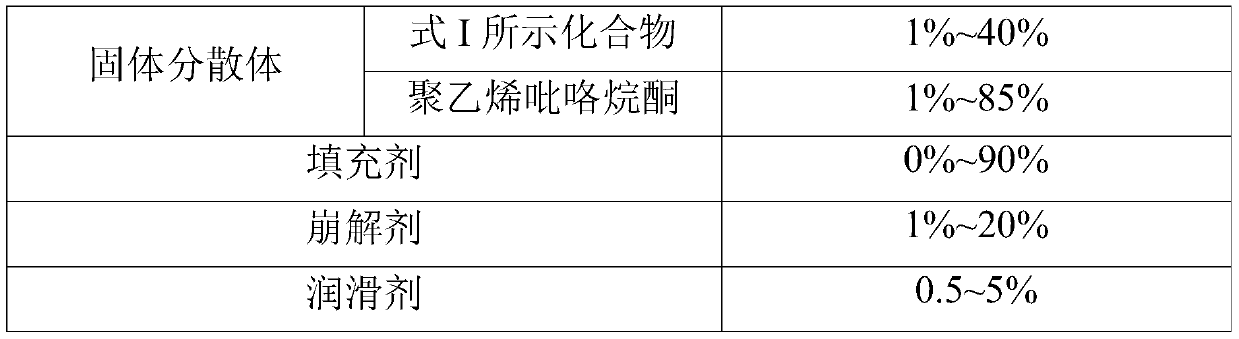
Pharmaceutical composition comprising PARP inhibitor
A technology of composition and medicine, applied in the field of pharmaceutical composition containing PARP inhibitors and its preparation, capable of solving problems such as poor stability of solid dispersions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
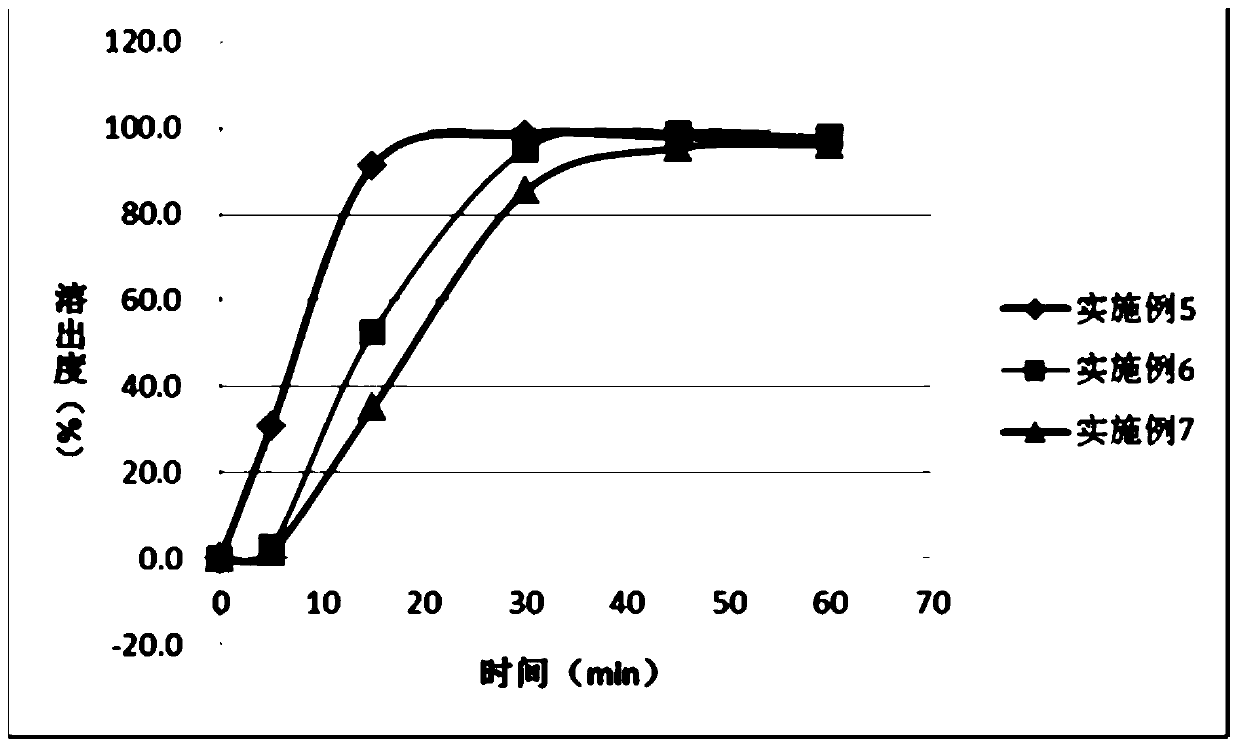
Examples
Embodiment 1
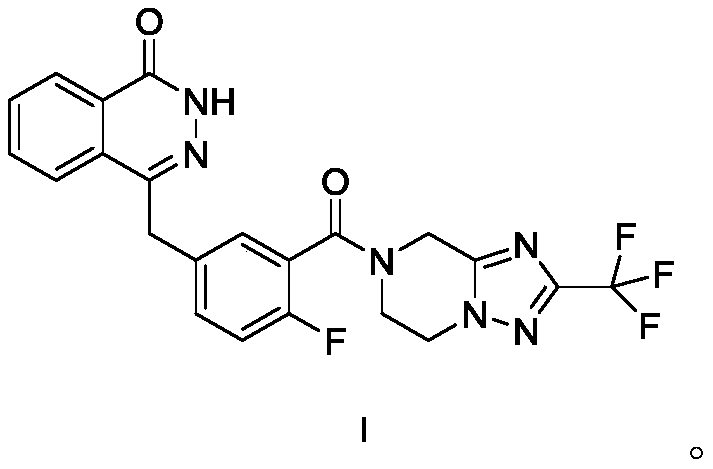
[0053] Referring to the general test in the second part of the Chinese Pharmacopoeia 2010 edition, weigh the excess compound shown in formula I that has been ground into a fine powder in a certain amount of solvent (25°C ± 2°C), shake vigorously for 30 seconds every 5min, and observe the concentration within 30min. For drug dissolution, filter, discard the primary filtrate and take the subsequent filtrate to detect the concentration and calculate the solubility.
[0054]
[0055] As can be seen from the test results, the compound shown in formula I is slightly soluble in acetone and tetrahydrofuran, very slightly soluble in absolute ethanol, almost insoluble in conventional aqueous solvents, and the solubilizing effect of surfactants on raw materials is limited, Therefore, preparations cannot be prepared by conventional means.
Embodiment 2-3
[0057]
[0058]
[0059] Remarks: *Solvents are removed during production
[0060] Add the prescribed amount of copovidone S630 or PVP K30 to ethanol to dissolve evenly, add the prescribed amount of acetone, stir and mix evenly, add the compound shown in formula I to the above solution, heat and stir at 60°C until the compound shown in formula I completely dissolved. The above solution was spray-dried, and the obtained powder was dried at 60° C. to obtain a solid dispersion.
Embodiment 4
[0062] Investigate respectively the physical stability of embodiment 2 and 3 two kinds of solid dispersions under high temperature (40 ℃, 60 ℃) and high humidity (25 ℃, RH75 ± 5%, 25 ℃, RH90 ± 5%) conditions and accelerate Chemical stability under conditions. Compare the appearance and crystal form changes of the two placed in a watch glass. The two solid dispersions were additionally tested for solvent residues.
[0063] Residual solvent detection method: adopt gas chromatography, DB-5 capillary column (30m×0.53mm×1.0μm), flame ionization detector (FID), and use water as solvent.
[0064] Active substance content, total impurities, etc. detection method (the same below): detection by high performance liquid chromatography system, the chromatographic column is Phenomenex Luna C18 column, 4.6mm×200mm, 5μm, mobile phase: 0.02mol / L potassium dihydrogen phosphate solution / acetonitrile, detection wavelength: 254nm, 200nm.
[0065] Influencing factor test
[0066]
[0067] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com