Cymipristone solid preparation and preparation methods thereof
A technology of cymipristone and solid preparations, which is applied in the field of cymipristone solid preparations and its preparation, and can solve problems such as environmental pollution, large loss, and unsatisfactory dissolution characteristics of cymipristone solid pharmaceutical preparations.
Active Publication Date: 2011-06-29
SHANGHAI ZHONGXI PHARMA +1
View PDF1 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0008] The technical problem to be solved by the present invention is to overcome the existing method for preparing cymipristone solid preparations by selecting and controlling the particle size of cymipristone through mechanical crushing, which will cause environmental pollution, large loss, and serious potential safety hazards. And the dissolution characteristics of cymipristone solid pharmaceutical preparations are still not ideal enough defects, and provide easier operation, less pollution, no aforementioned safety hazards, and can significantly improve the dissolution characteristics of the obtained cymipristone solid preparations, and ensure better A preparation method with good stability and content uniformity, and a solid preparation of cymipristone prepared by the method
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
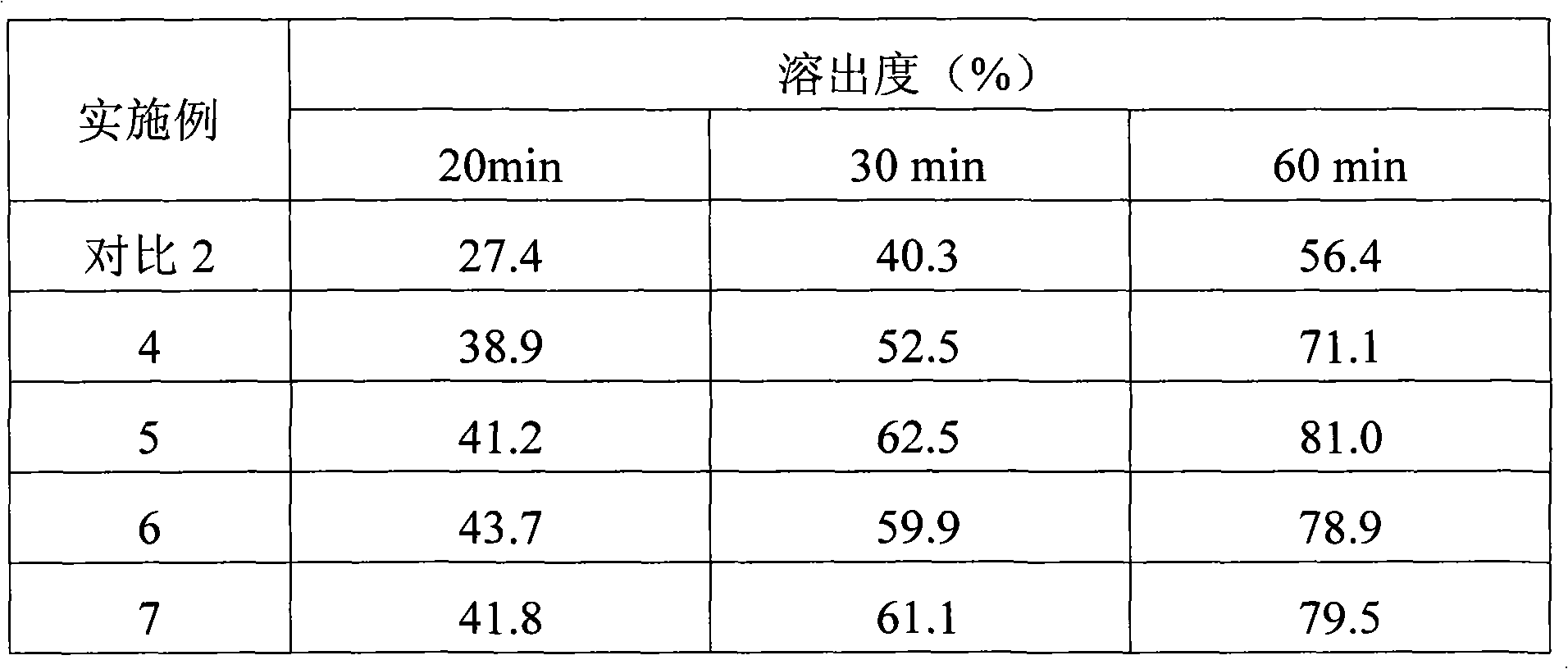
Experimental program
Comparison scheme
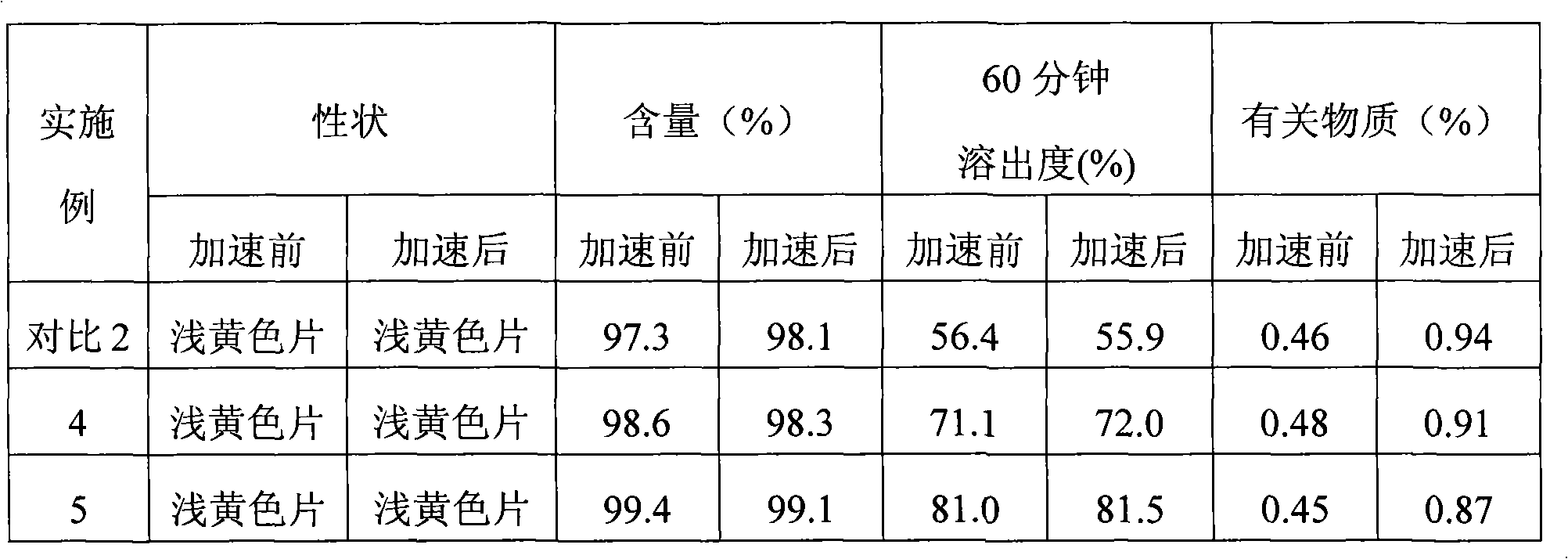
Effect test
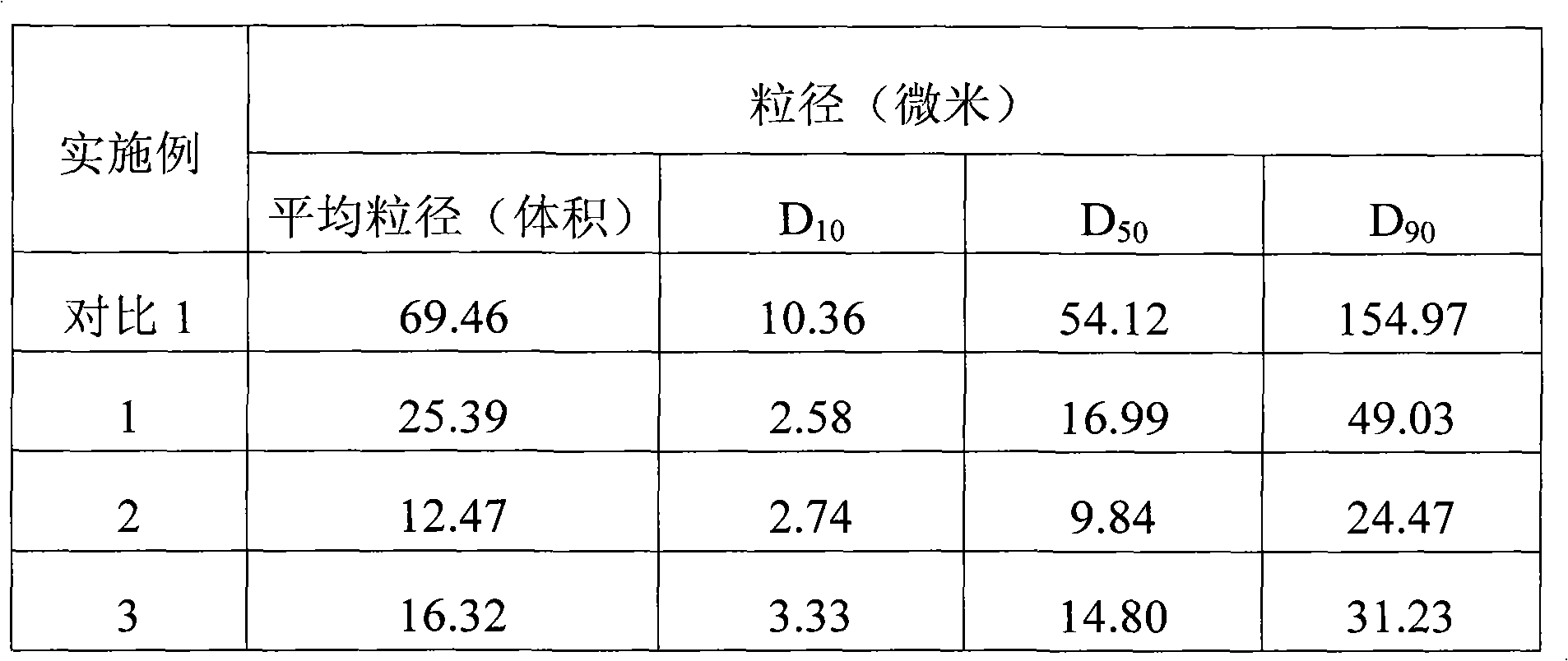
Embodiment 1~2
[0059] Embodiment 1~2 formula and preparation method of cymipristone granules
[0060]
Embodiment 5
[0066] Embodiment 5 Cymipristone tablet (5mg / tablet) formula and preparation method
[0067] drug
[0068]
Embodiment 6
[0069] Embodiment 6 Cymipristone tablet (5mg / tablet) formula and preparation method
[0070] drug
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses preparation methods of a cymipristone solid preparation. A method 1 comprises the following steps: dissolving cymipristone in a solvent to obtain a drug-containing solution, then evenly mixing auxiliary materials with the drug-containing solution, and performing wet granulation, wherein, the solvent is an organic solvent or an aqueous solution containing more than 85% of an organic solvent by mass percent. A method 2 comprises the following steps: dissolving the cymipristone in an acid solution containing an acidulant to obtain a drug-containing acid solution, then evenly mixing the auxiliary materials with the drug-containing acid solution, and performing wet granulation. The invention further discloses the cymipristone solid preparation prepared by the methods. The preparation methods disclosed by the invention avoid the defects such as serious pollution, great loss and serious potential safety hazard caused by mechanical pulverization, and the methods are simple and feasible in operation and have a high safety factor, thus being applicable to industrial production. The cymipristone solid preparation prepared by the methods has the advantages of excellent leaching property, better stability and better content uniformity.
Description
technical field [0001] The invention belongs to the field of pharmaceutical preparations, and in particular relates to a solid preparation of cymipristone and a preparation method thereof. Background technique [0002] Cymipristone belongs to steroidal compounds, chemical name: 11β-(4-(N-methyl-N-cyclohexylamino)phenyl)-17β-hydroxy-17α-(1-propynyl)-estr Ste-4,9-dien-3-one, with a molecular weight of 497.71, has dual effects of progesterone receptor antagonism and glucocorticoid receptor antagonism. Chinese patent document CN1287123 discloses its preparation method and its pharmaceutical composition and application. [0003] Cymipristone is a weak base drug and is insoluble in water. When preparing solid preparations, cymipristone needs to be pulverized to a certain fineness to ensure that the solid preparations can dissolve rapidly after oral administration. At present, mechanical pulverization is generally used for the pulverization of poorly water-soluble drugs. Howeve...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/00A61K9/16A61K31/565A61P5/36A61P43/00
Inventor 郑斯骥谭波袁少卿傅麟勇
Owner SHANGHAI ZHONGXI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com