Method for preparing solid preparation and solid preparation
A solid preparation and solvent technology, used in pill delivery, pharmaceutical formulations, antiviral agents, etc., can solve the problems of unsatisfactory dissolution characteristics of solid pharmaceutical preparations, potential safety hazards, environmental pollution, etc., achieving small individual differences, high safety factor, Avoid polluting effects
Active Publication Date: 2011-06-29
SHANGHAI ZHONGXI PHARMA +1
View PDF0 Cites 21 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0008] The technical problem to be solved by the present invention is to overcome the existing solid preparation method to select and control the particle size of the pharmaceutical active ingredient through mechanical pulverization, which will cause environmental pollution, serious safety hazards, large loss, and the resulting solid pharmaceutical preparation However, for water-insoluble or poorly water-soluble basic drugs, it is easier to operate, less polluted, without the aforementioned safety hazards, and can ensure that the prepared solid preparation has excellent dissolution characteristics. , preparation method of stability and content uniformity and obtained solid preparation
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 12
[0041] Example 12 Formula and preparation method of eszopiclone tablets (2mg / tablet)
[0042]
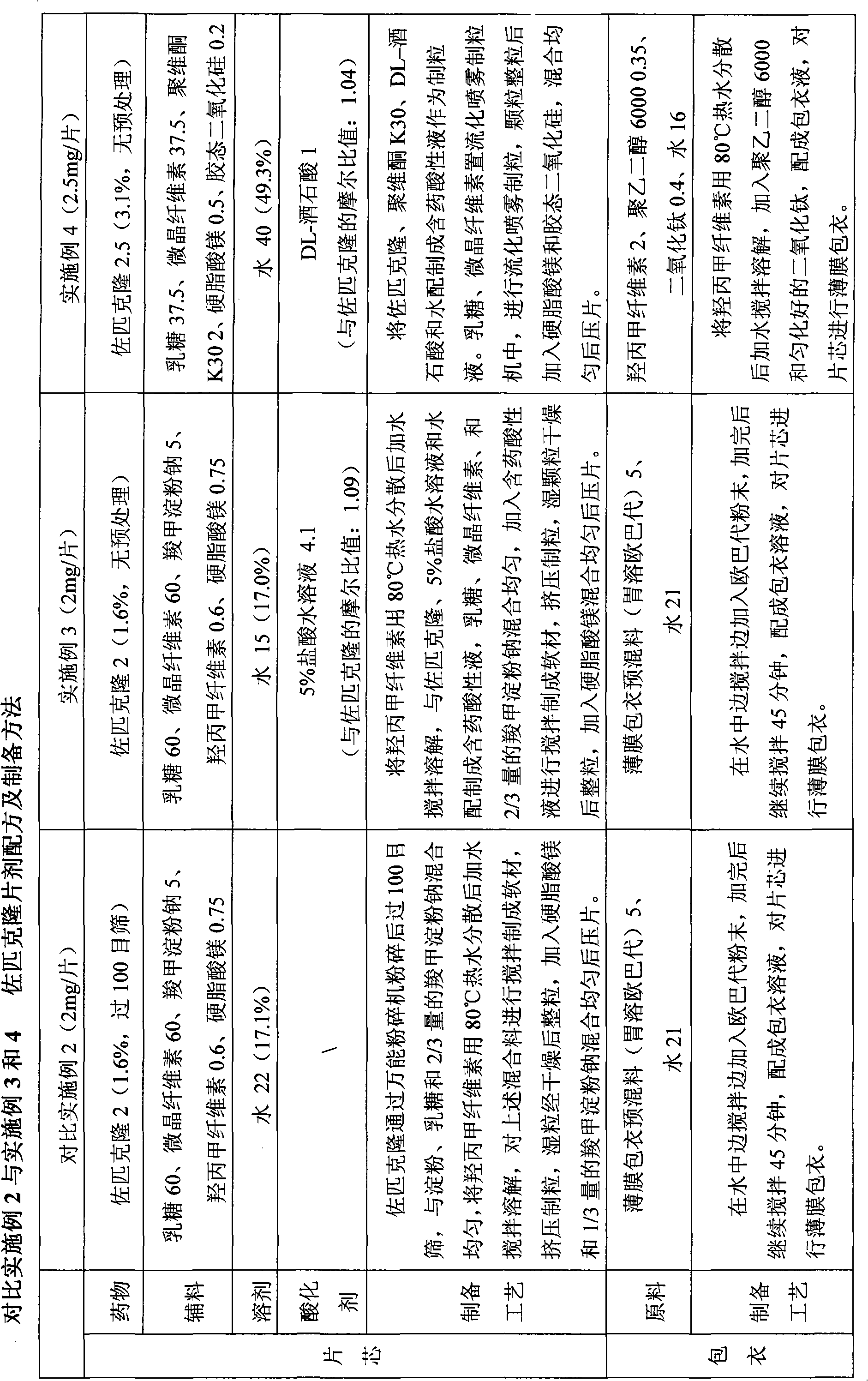
Embodiment 13
[0043] Example 13 Eszopiclone Tablets (2mg / tablet) Formula and Preparation Method
[0044]
Embodiment 14
[0045] Example 14 Eszopiclone Tablets (2mg / tablet) Formula and Preparation Method
[0046]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Login to View More
Abstract
The invention discloses a method for preparing a solid preparation, which comprises the following steps of: dissolving active ingredients of water-insoluble and / or lowly water-soluble alkaline medicaments in acidulant-containing acid solution to prepare medicament-containing acid solution; and mixing the medicament-containing acid solution and auxiliary materials uniformly to perform wet-method pelletizing. The invention also discloses the solid preparation prepared by the method. By the method, the defects of serious pollution, large loss and serious potential safety hazard which are caused by mechanical pulverization are overcome, and the method is easy and convenient to operate, has high safety factors and is applied to industrial production easily. The solid preparation prepared by the method has the excellent dissolution characteristic, stability and content uniformity.
Description
technical field [0001] The invention belongs to the field of pharmaceutical preparations, and in particular relates to a preparation method of a solid preparation and the obtained solid preparation. Background technique [0002] In the field of pharmaceutical preparations, the particle size of active pharmaceutical ingredients is closely related to the preparation process and quality of solid preparations. In the preparation process of specific pharmaceutical preparations, the particle size of the active pharmaceutical ingredients is usually selected according to the dissolution characteristics of the medicine and the permeability of the biomembrane. For example, if it is a drug with poor solubility and drug dissolution is the rate-limiting process of absorption, a smaller particle size can be selected to promote drug absorption. For another example, if it is a drug with poor compressibility, its compressibility can be improved by selecting an appropriate particle size and ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/00A61K9/16A61K47/02A61K47/12A61K47/20A61K47/26A61K47/32A61K47/34A61K47/38A61K47/40A61K47/44A61K31/496A61K31/4985A61K31/519A61P7/02A61P25/18A61P25/20A61P31/12
CPCA61K31/551A61K31/475A61K31/519A61K31/5513A61K31/506A61K31/165A61K31/454A61K47/02A61K47/12A61K31/496A61K9/1617A61K9/2009A61K31/4515A61K31/4985A61K9/2013A61K31/5517A61K31/567A61K31/5415A61K31/4164A61K31/7048A61K9/1611A61K31/166A61P7/02A61P25/18A61P25/20A61P31/12
Inventor 郑斯骥谭波
Owner SHANGHAI ZHONGXI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com