Carvedilol push-pull osmotic pump type controlled release preparation and preparation method thereof
A technology of controlled-release preparations and osmotic pumps, which is applied in pharmaceutical formulations, pill delivery, and medical preparations containing active ingredients. problems, to achieve the effect of reducing the number of medications, improving curative effect and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Tablet core drug layer prescription
[0039]
[0040] Tablet core booster layer prescription
[0041]
[0042] Preparation method of tablet core:
[0043] 1. Take carvedilol through an 80 mesh sieve;
[0044] 2. Weigh the main medicine and related auxiliary materials according to the prescription, mix them evenly, add an appropriate amount of 95% ethanol to make the soft material, granulate with a 20-mesh sieve, and dry completely at 40°C with air blowing;
[0045] 3. After the granules are completely dried, use an 18-mesh sieve to size them, add the prescribed amount of magnesium stearate and mix evenly to obtain granules A for use.
[0046] 4. Weigh the auxiliary materials according to the formula of the booster layer, mix them, add an appropriate amount of 100% ethanol to prepare soft materials, granulate with 20 mesh sieve, and dry completely at 40°C; pass through 18 mesh sieve and add the prescribed amount of hard fat Magnesium acid, get granule B for use.
[0047] 5. Select ...
Embodiment 2
[0056] Tablet core prescription:
[0057]
[0058] Tablet core booster layer prescription:
[0059]
[0060] Coating liquid prescription (200ml coating liquid dosage):
[0061]
[0062] According to the tablet core prescription, the tablet core was prepared according to the tablet core preparation method of Example 1. According to the coating liquid prescription, the Carvedil of this example was prepared according to the conventional film coating method and the same aging method and perforating method of Example 1. Luo osmotic pump piece finished product.
Embodiment 3
[0064] Tablet core prescription:
[0065]
[0066]
[0067] Tablet core booster layer prescription
[0068]
[0069] Coating liquid prescription (200ml coating liquid dosage)
[0070]
[0071] According to the tablet core prescription, the tablet core was prepared according to the tablet core preparation method of Example 1. According to the coating liquid prescription, the Carvedil of this example was prepared according to the conventional film coating method and the same aging method and perforating method of Example 1. Luo osmotic pump piece finished product.
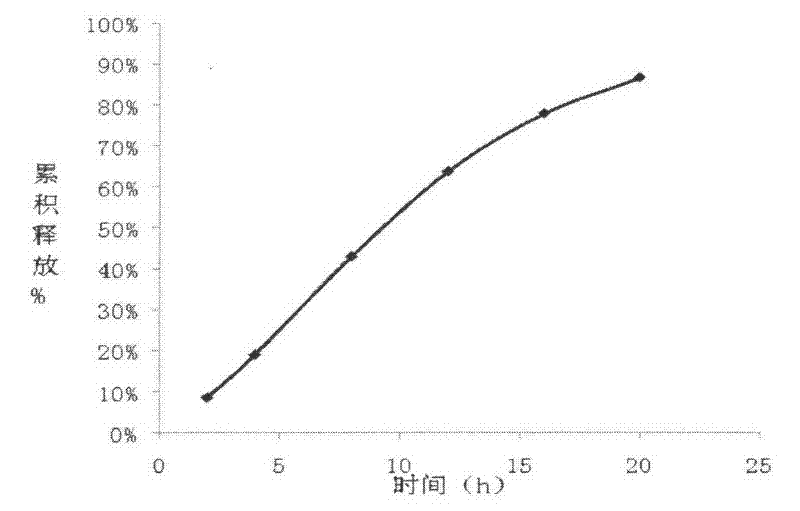
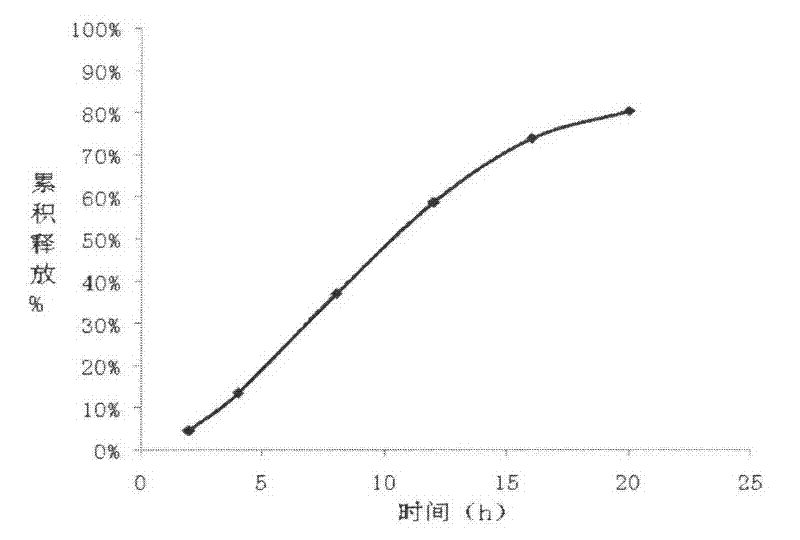
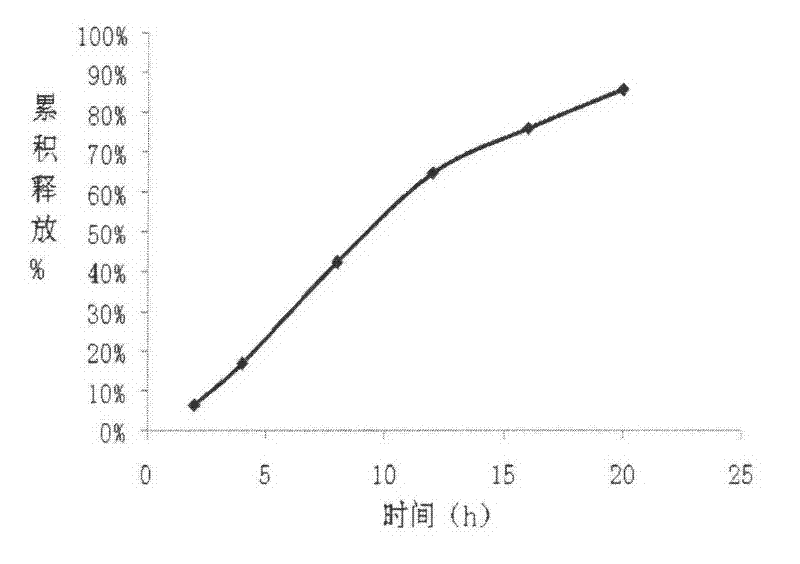
[0072] Release determination:
[0073] Take the sample and use the device for the irradiance determination method (the first method of appendix XD of the Chinese Pharmacopoeia 2005 edition) and the device of the dissolution test method (the second appendix XC method of the Chinese Pharmacopoeia 2005 edition) with a hydrochloric acid solution (9→1000 ) 900ml is the dissolution medium, the measuring temperature is (37±0.5)℃, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com