Carvedilol sulphate crystals, preparation method and application thereof in medicine
A technology of carvedilol and sulfuric acid, applied in the field of congestive heart failure and angina pectoris, preparation and treatment of high blood pressure, can solve problems that do not involve research on crystal forms and their preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
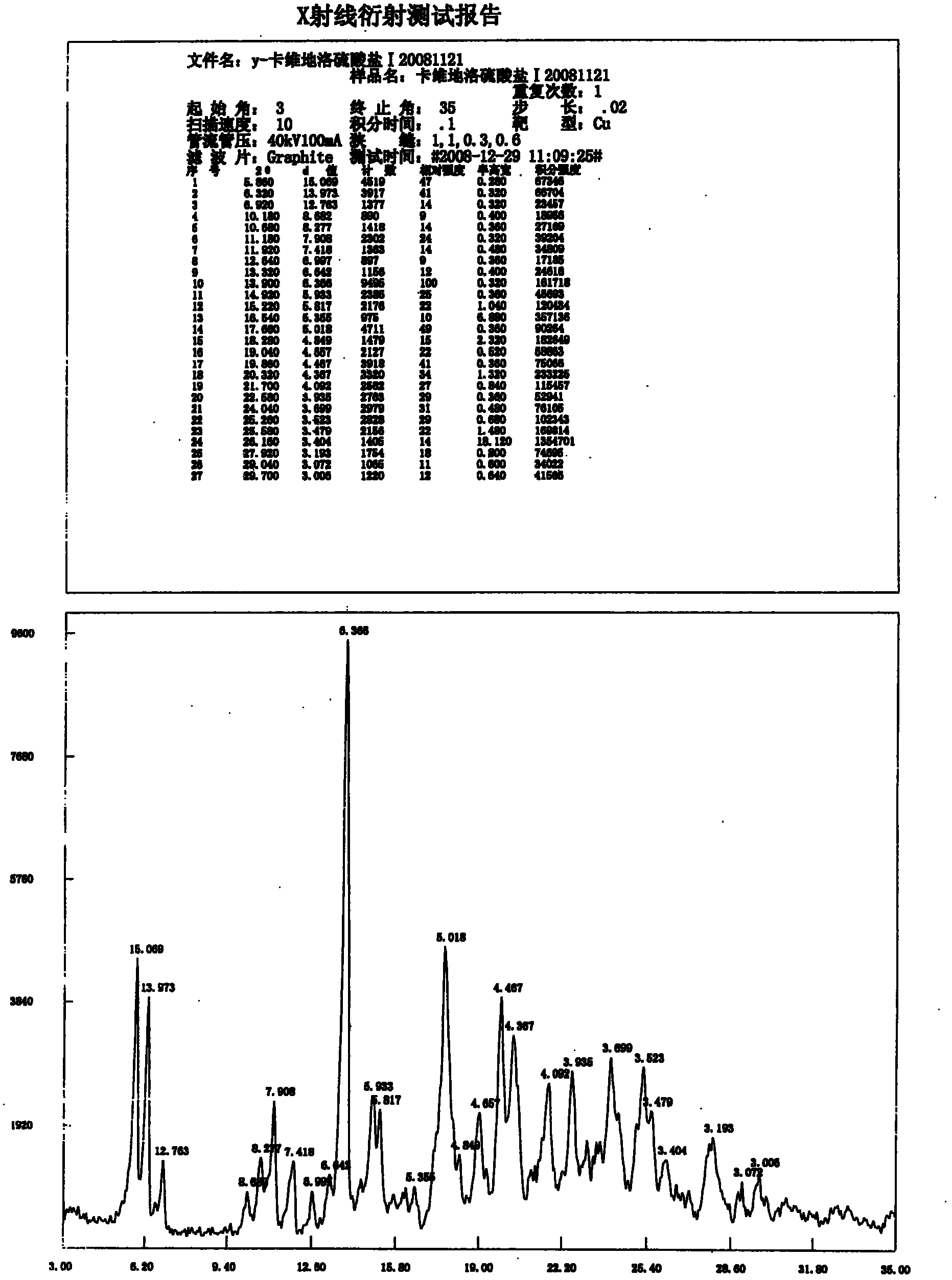
[0060] Carvedilol (synthesized according to patent US4503067 (1983)) 25g (0.062mol) is dissolved in 250ml ethanol, and heating makes it dissolve, and sulfuric acid (0.031mol, 3.04g, the concentrated sulfuric acid of 1.68ml98%) is diluted in 30ml of water. Slowly added dropwise to carvedilol ethanol solution, a white solid precipitated, and continued to stir overnight. The next day, it was filtered with suction and dried under reduced pressure at 60° C. for 3 hours to obtain 25.8 g of carvedilol sulfate as a white solid with a water content of 2.29%. The molar yield is 92.1%.
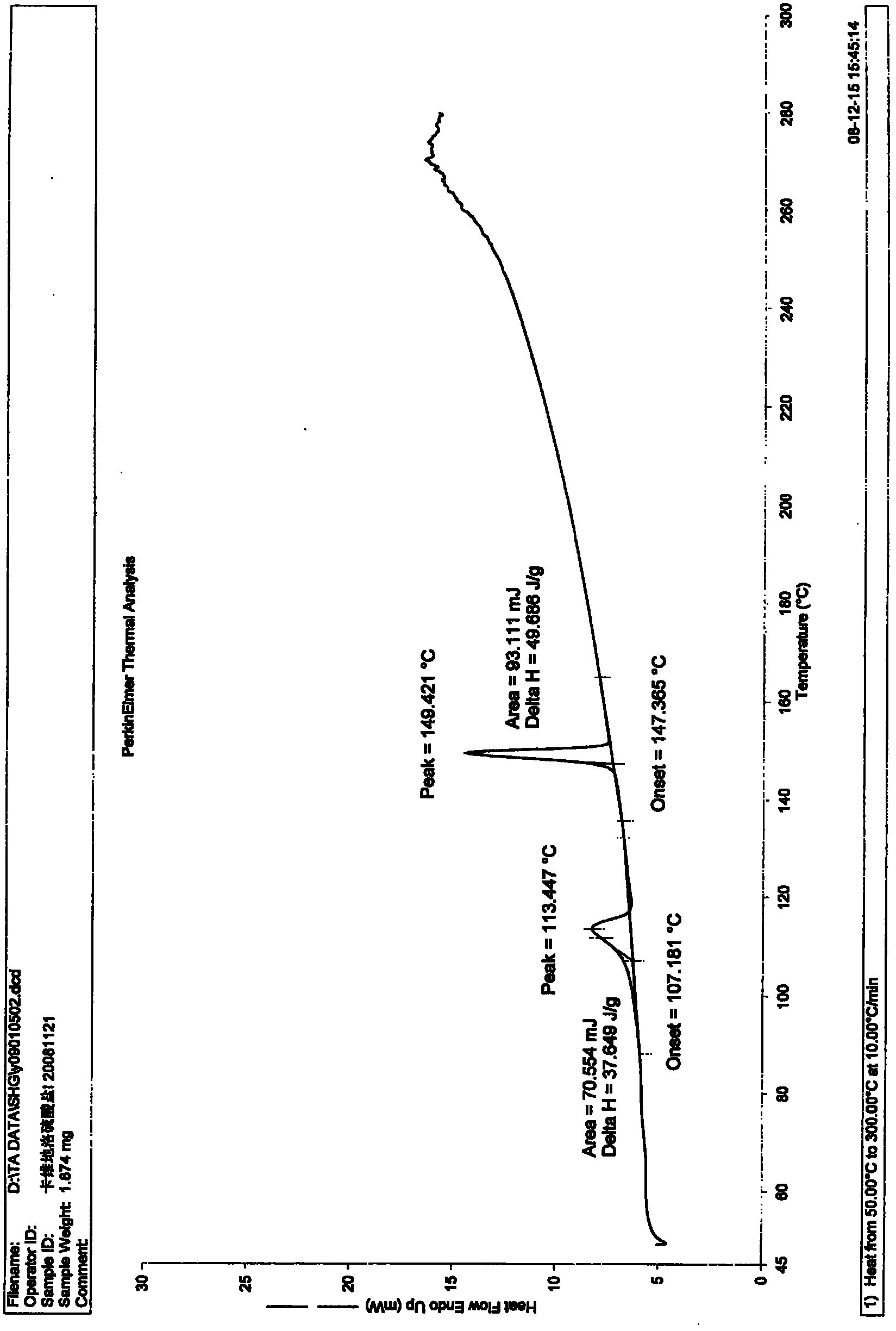
[0061] Add 2.0 g (2.2 mmol) of carvedilol sulfate prepared in the previous step into 65 ml of 95% ethanol solution, heat and reflux to clarification, keep for 30 minutes, stir and cool to room temperature for crystallization, filter, and the obtained crystals are crystallized at 60 °C for 3 hours in vacuum to obtain 1.86 g of crystals with a mass yield of 93% and a water content of 2.63%. The X-ray di...
Embodiment 2
[0063] Carvedilol sulfate 2.0g (2.2mmol) prepared in Example 1 was added to 250ml of water, heated to reflux until clarified, slowly cooled to room temperature for crystallization, filtered, and the obtained crystals were vacuum-dried at 60°C for 3 hours, 1.87 g of crystals were obtained, the water content was 2.73%, and the mass yield was 93.5%.
Embodiment 3
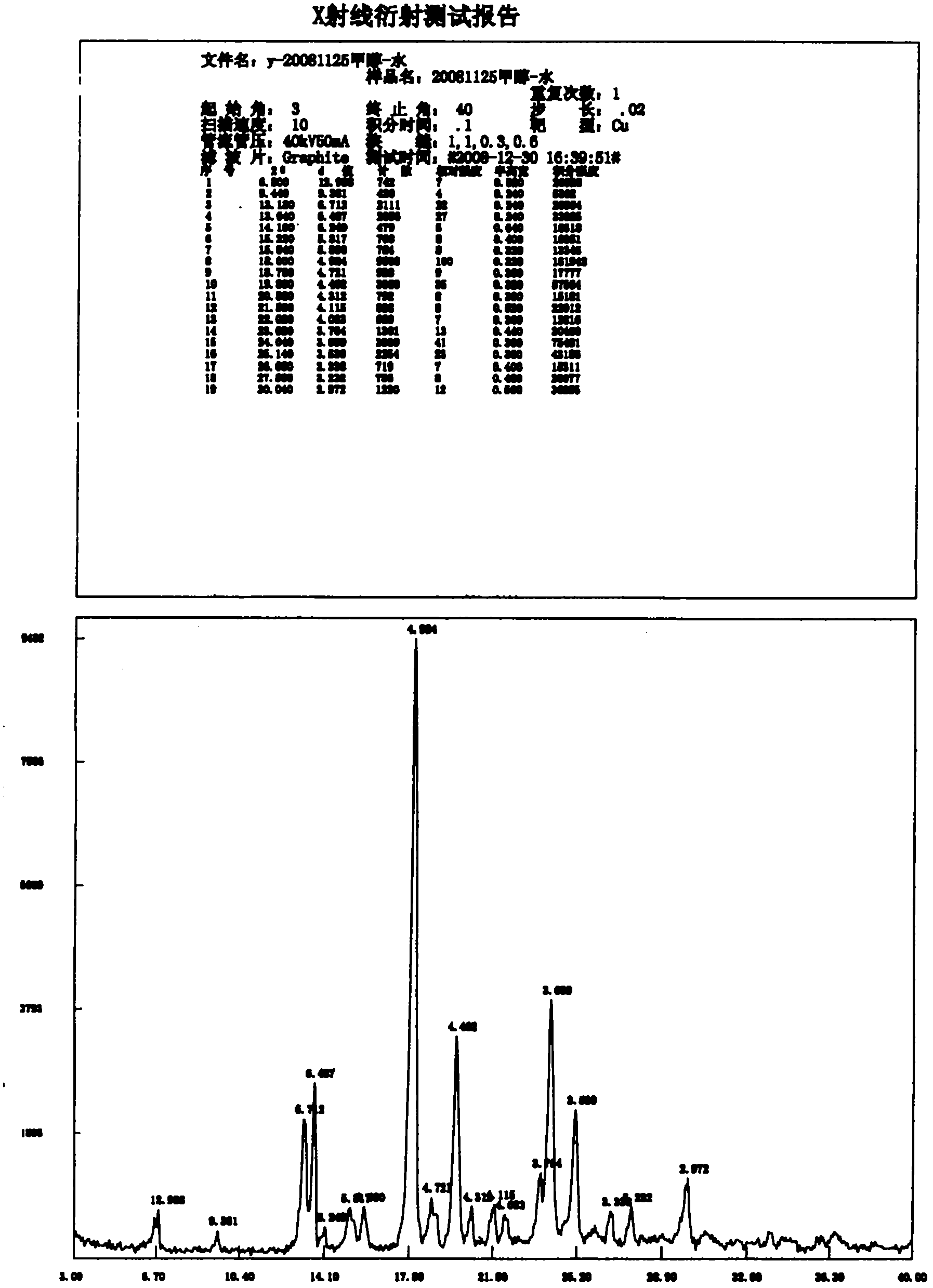
[0065] Add 2.0 g (2.2 mmol) of carvedilol sulfate prepared in Example 1 into 25 ml of 50% acetone aqueous solution, heat to reflux until clarification, keep for 30 minutes, slowly cool to room temperature for crystallization, filter, and the obtained crystal Vacuum dried at 60° C. to obtain 1.72 g of crystals, with a mass yield of 85.9% and a water content of 2.59%. The characteristic absorption of the X-ray diffraction spectrum of the crystalline sample is the same as that of the A-type crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com