Carvedilol free base, salts, anhydrous forms or solvates thereof, corresponding pharmaceutical compositions, controlled release formulations, and treatment or delivery methods
a technology of carvedilol and free base, which is applied in the direction of biocide, drug composition, cardiovascular disorder, etc., can solve the problems of limiting the residence time of the intestine, causing the intestine to be a considerable problem, and affecting the intestine or inability to maintain the chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
embodiments
[0436] In light of the foregoing discussion, the present invention relates to and is exemplified by, but not limited to the following embodiments present below, which include corresponding pharmaceutical compositions, different controlled release formulations, respectively comprised or formed from the following components, such as carvedilol free base, carvedilol salts, anhydrous forms or solvates thereof, and which also may include, but are not limited to the various components (i.e., such as conventionally known, adjuvants, carriers, diluents, excipients, agents, plasticizers, polymers, etc. as described herein) which may be in or formed into different dosage forms (i.e., which may include, but are not limited to, tablets, capsules and the like) as described herein.
[0437] In a general or first embodiment, the present invention relates to a controlled release formulation, which comprises: [0438] a solubility enhanced carvedilol free base or a carvedilol salt, solvate or anhydrous ...
example 1
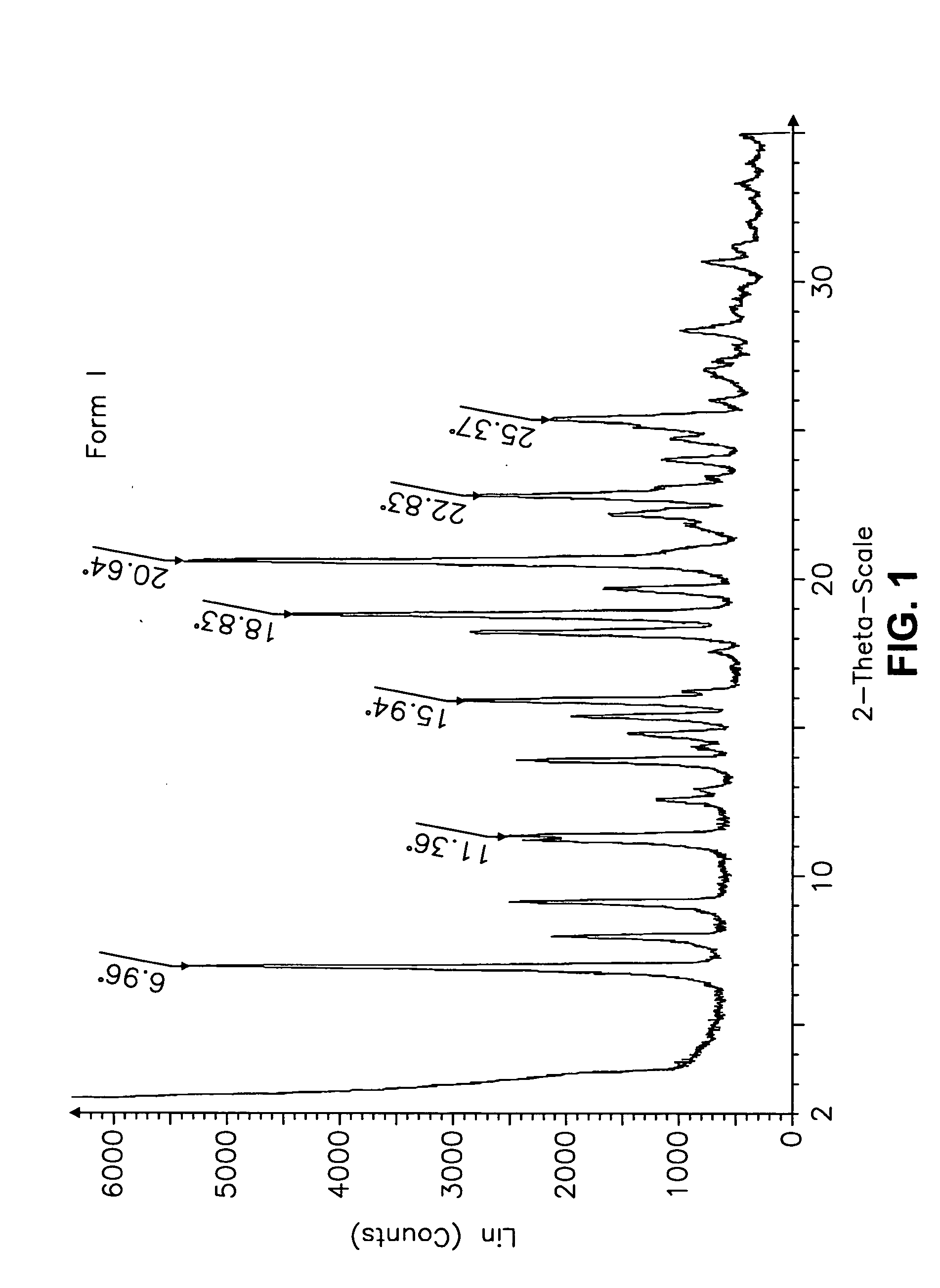
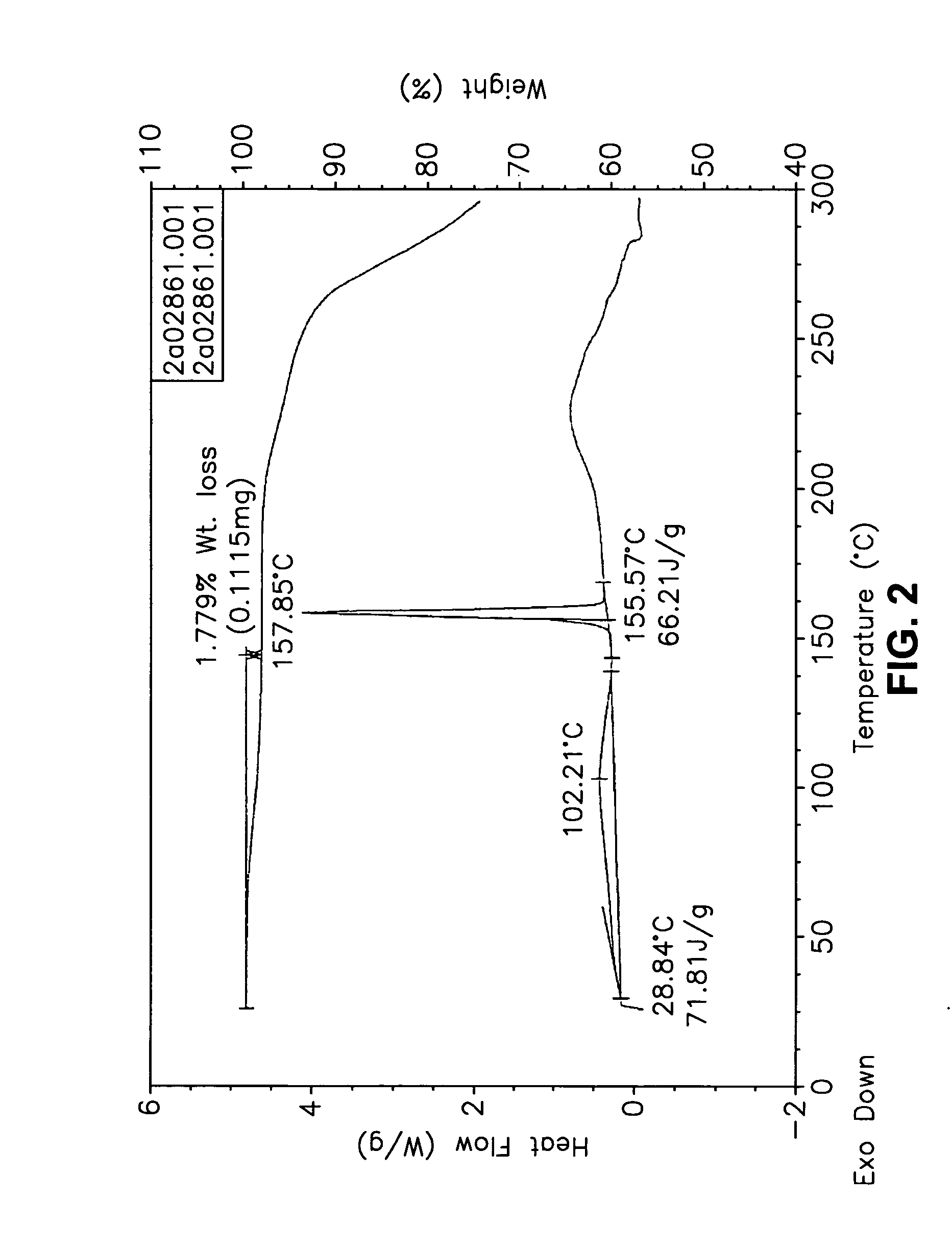
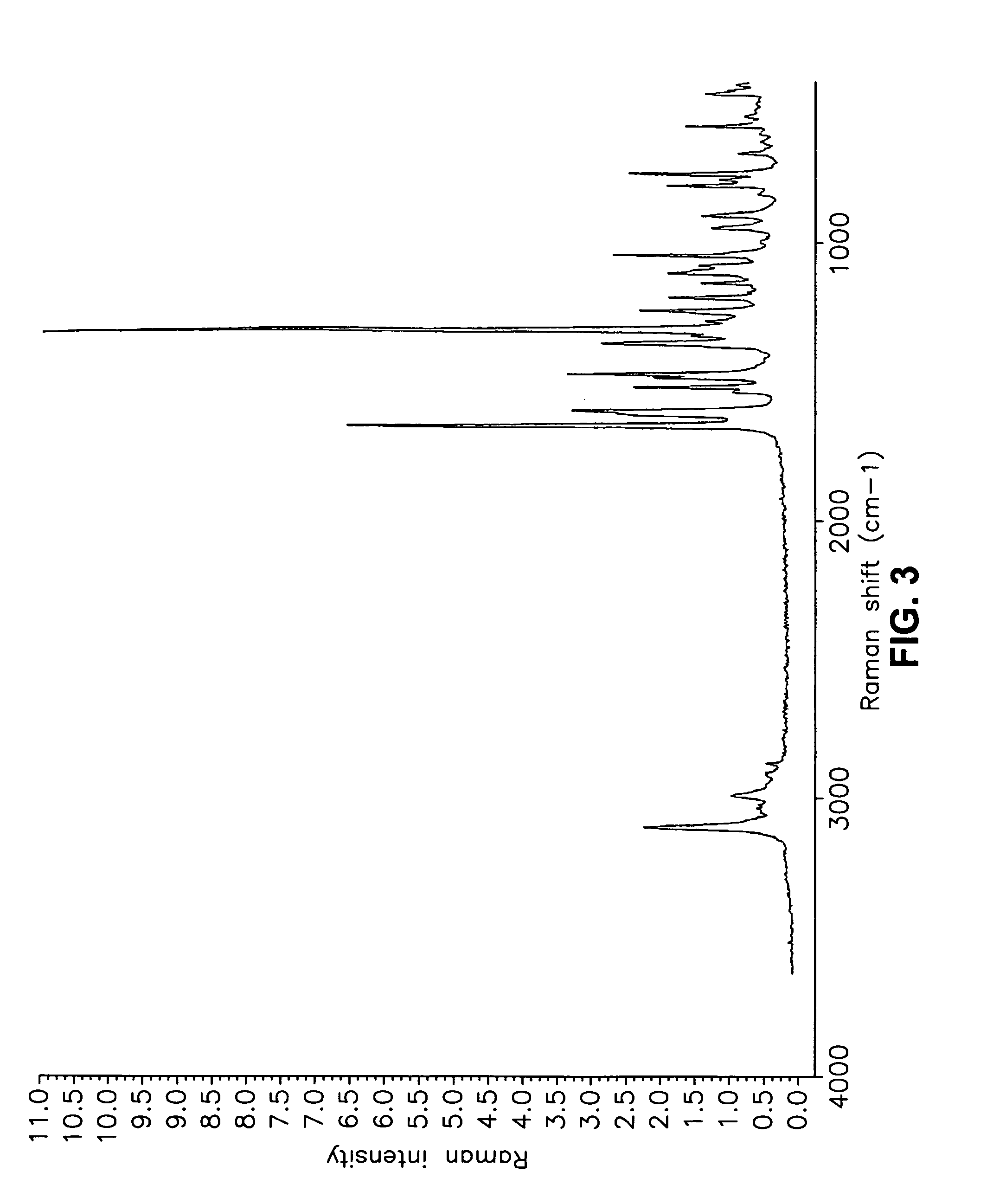
Form I Carvedilol Dihydrogen Phosphate Hemihydrate Preparation
[0627] A suitable reactor is charged with acetone. The acetone solution is sequentially charged with carvedilol and water. Upon addition of the water, the slurry dissolves quickly. To the solution is added aqueous H3PO4. The reaction mixture is stirred at room temperature and carvedilol dihydrogen phosphate seeds are added in one portion. The solid precipitate formed is stirred, then filtered and the collected cake is washed with aqueous acetone. The cake is dried under vacuum to a constant weight. The cake is weighed and stored in a polyethylene container.
example 2
Form II Carvedilol Dihydrogen Phosphate Dihydrate Preparation
[0628] Form I is slurried in acetone / water mixture between 10 and 30° C. for several days.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com