Levetiracetam slow release medicinal composite and preparation method thereof
A sustained-release drug and composition technology, applied in the field of medicine, can solve problems such as complex preparation process, and achieve the effect of simple preparation process, easy reproducibility, and low preparation equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
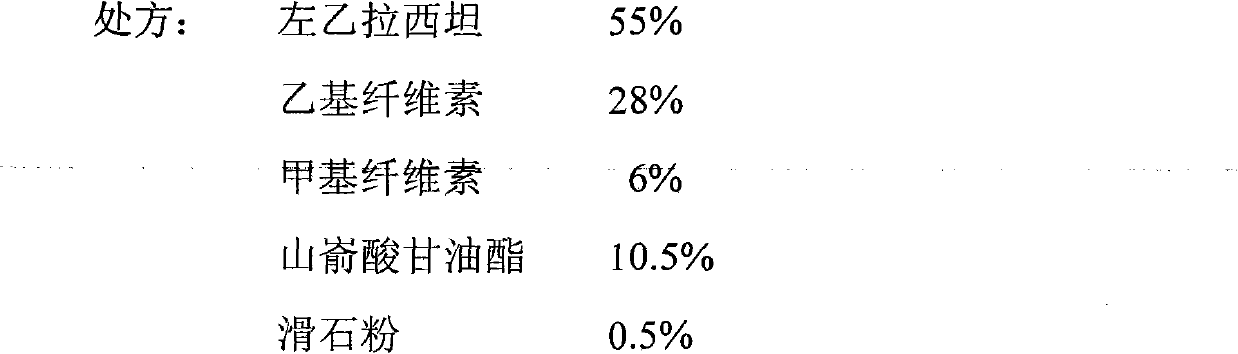
[0045]
[0046] Preparation method: Pass levetiracetam, ethyl cellulose, methyl cellulose and glyceryl behenate through a 60-mesh sieve, mix evenly according to the above ratio, then dry granulate, pass through a 20-mesh sieve for granulation, add the above-mentioned Proportional talcum powder is mixed, compressed into tablets, and the tablet core is coated with an aqueous dispersion of 5% OPADRY (W / V), and the weight of the tablet after coating is increased by 2% compared with that before coating.
Embodiment 2
[0048]
[0049] Preparation method: pass levetiracetam, ethyl cellulose, methyl cellulose, and glyceryl behenate through a 60-mesh sieve, mix uniformly according to the above ratio, and mix ethyl cellulose and 1% of the total weight of the above mixture with Concentration is the solution that the concentration that 95% ethanol configuration forms is 10% (W / W), this solution is added in the mixture, wet granulation, dry, cross 20 mesh sieves, granulate, add the magnesium stearate of above-mentioned ratio, Mix evenly, press into tablets, the tablet core is coated with 5% OPADRY (W / V) aqueous dispersion, the weight of the tablet after coating increases by 2.5% compared with that before coating.
Embodiment 3
[0051]
[0052] Preparation method: pass levetiracetam, ethyl cellulose, methyl cellulose, and glyceryl behenate through a 60-mesh sieve, mix evenly according to the above ratio, and mix PVP-K30 accounting for 0.5% of the total weight of the above mixture with no Water ethanol is configured into a solution with a concentration of 15% (W / W), and the solution is added to the mixture, then wet granulated, dried, passed through a 20 mesh sieve, and added glyceryl behenate accounting for 1% of the total weight of the dry granules With the magnesium stearate of above-mentioned ratio, mix, tabletting, tablet core use concentration is the coating of 6% OPADRY (W / V) water dispersion, the tablet after coating is compared with the weight gain of 2% before coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com