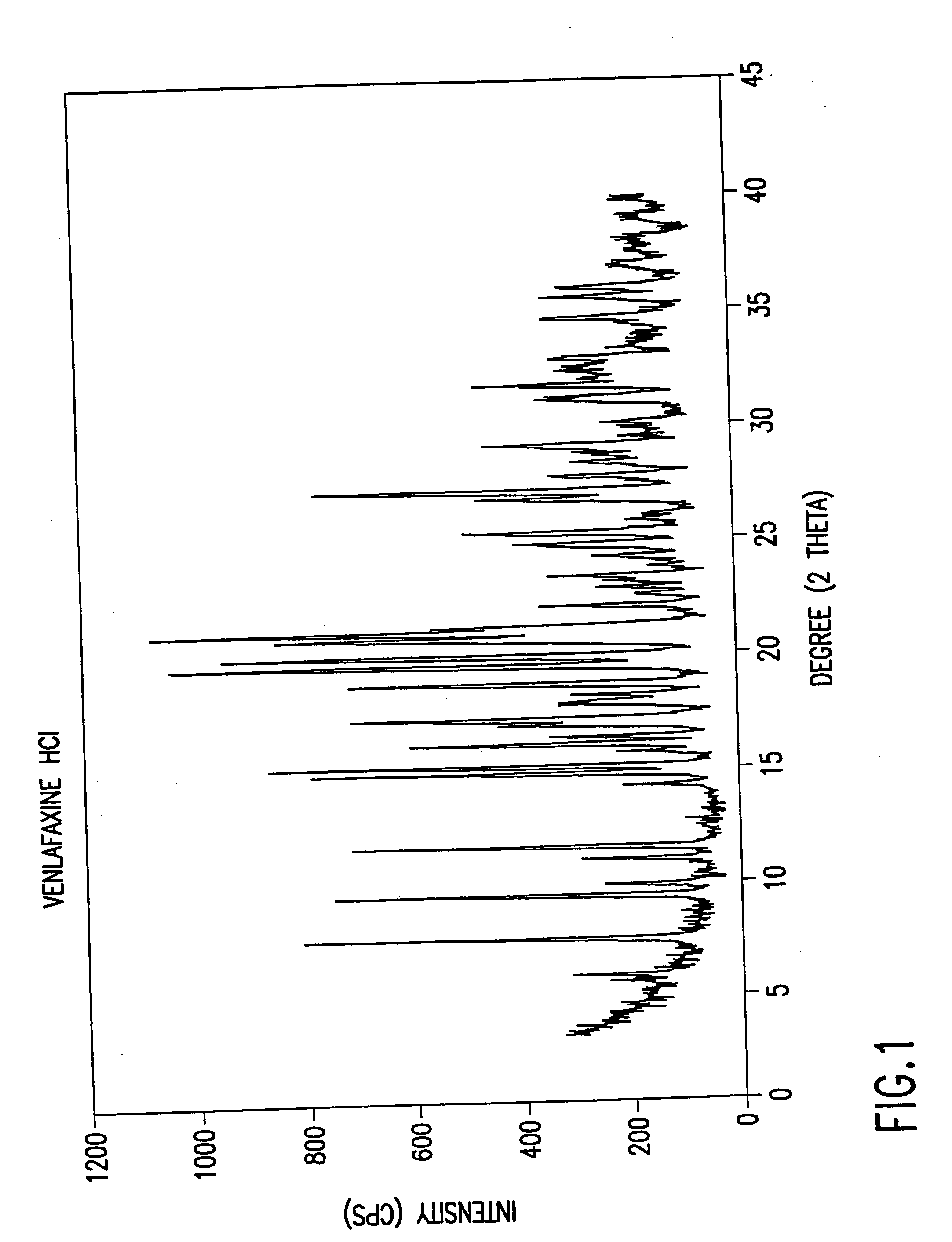
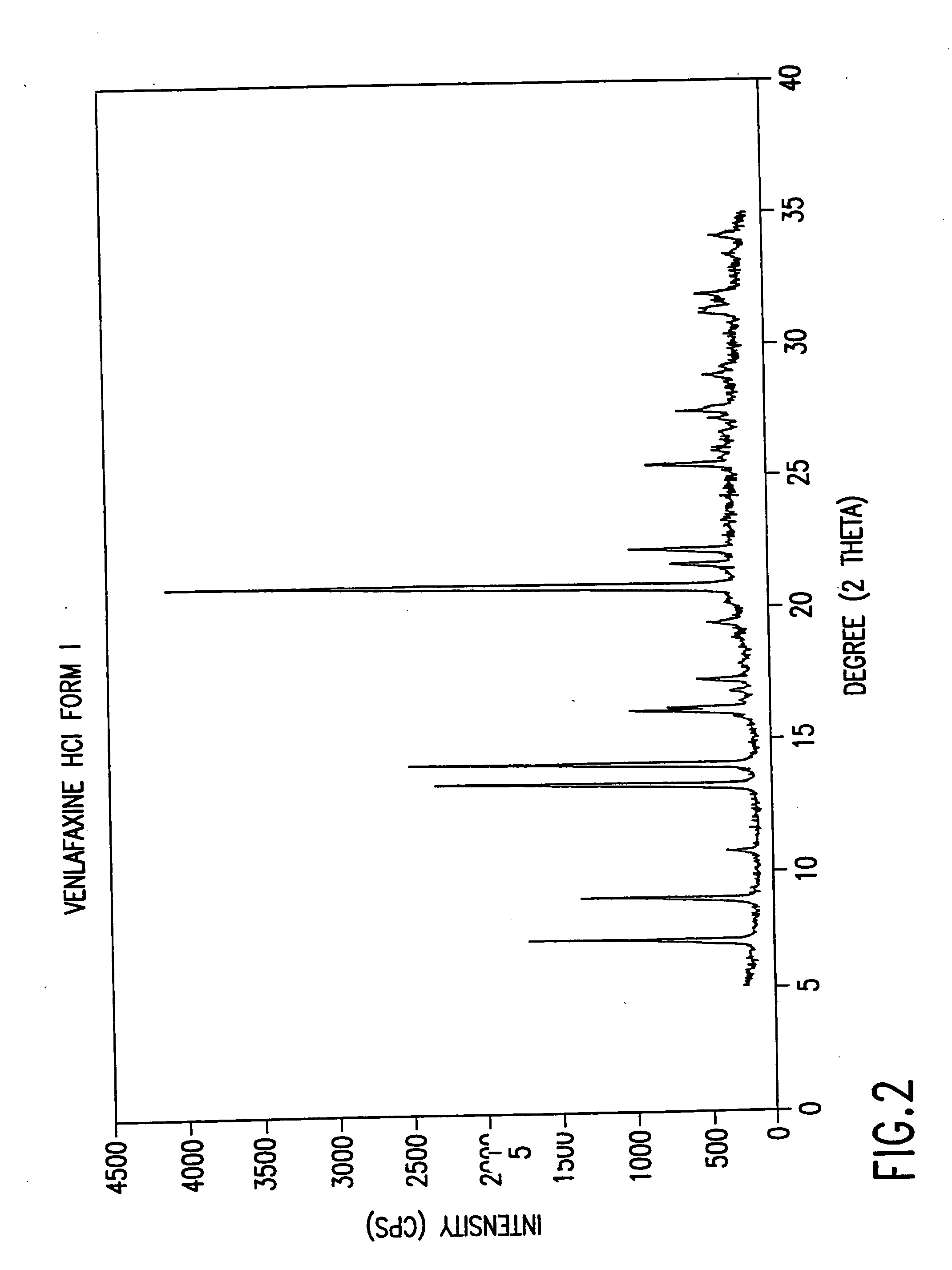
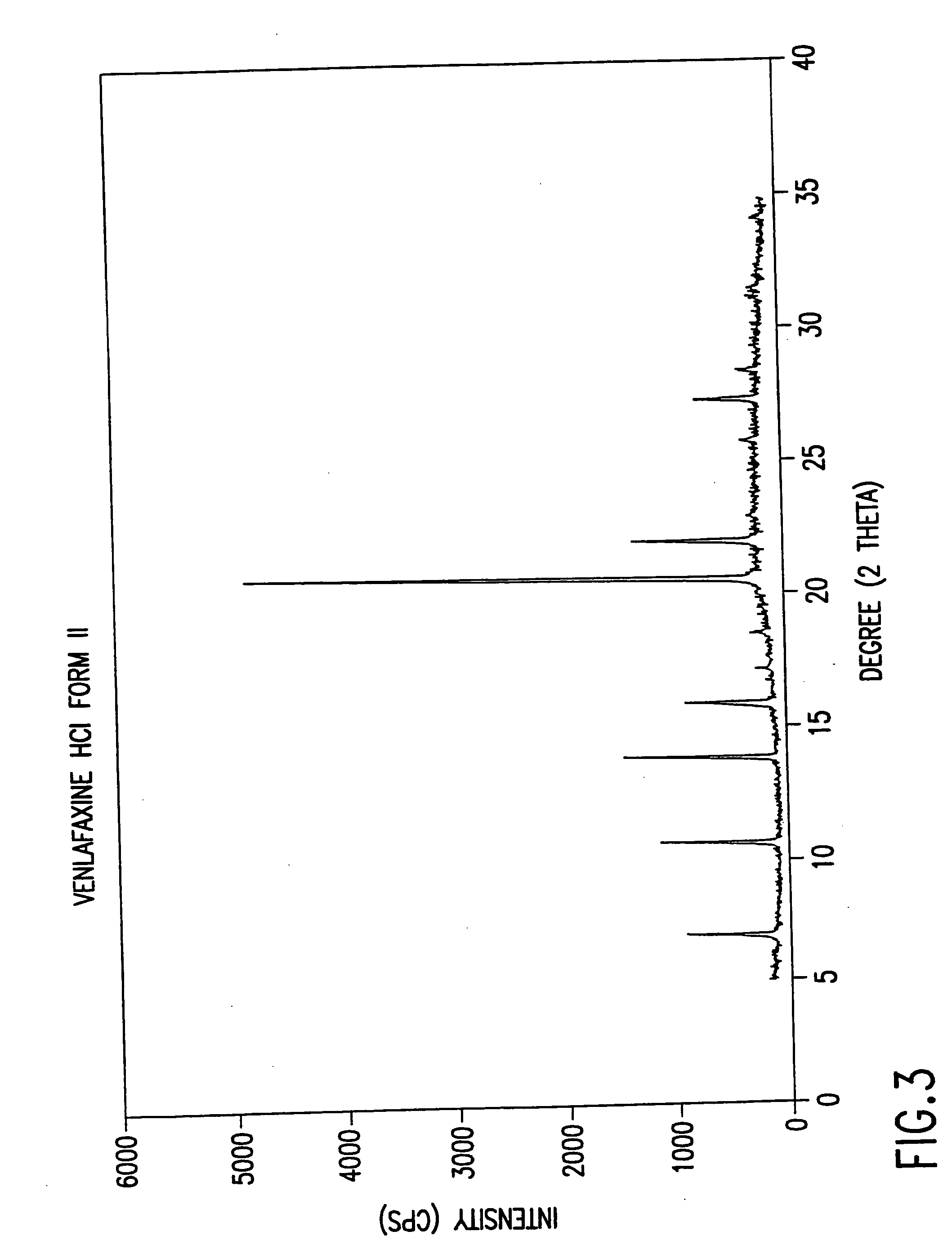
Novel crystalline polymorph of venlafaxine hydrochloride and methods for the preparation thereof
a technology of venlafaxine hydrochloride and crystalline polymorphism, which is applied in the field of new crystalline polymorphism of venlafaxine hydrochloride, can solve the problem of no inhibitory activity of monoamine oxidas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Anhydrous Venlafaxine Hydrochloride
[0039] About 100 mg of venlafaxine hydrochloride of Form II was put in a glass vial, flushed with nitrogen gas and then sealed by heat. The sealed vial was put in an oil bath at about 200° C. (197° C. to 200° C.) for 1 hour or until the shape of the crystals changed to form cream-white crystals.
example 2
Preparation of Anhydrous Venlafaxine Hydrochloride
[0040] The procedure in Example 1 was repeated with 500 mg of venlafaxine hydrochloride of Form II, except the vial was left in the oil bath for 2.5 hours instead of 1 hour.
example 3
Preparation of Anhydrous Venlafaxine Hydrochloride
[0041] The procedure in Example 1 was repeated, except an aluminum vial was used instead of a glass vial.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com