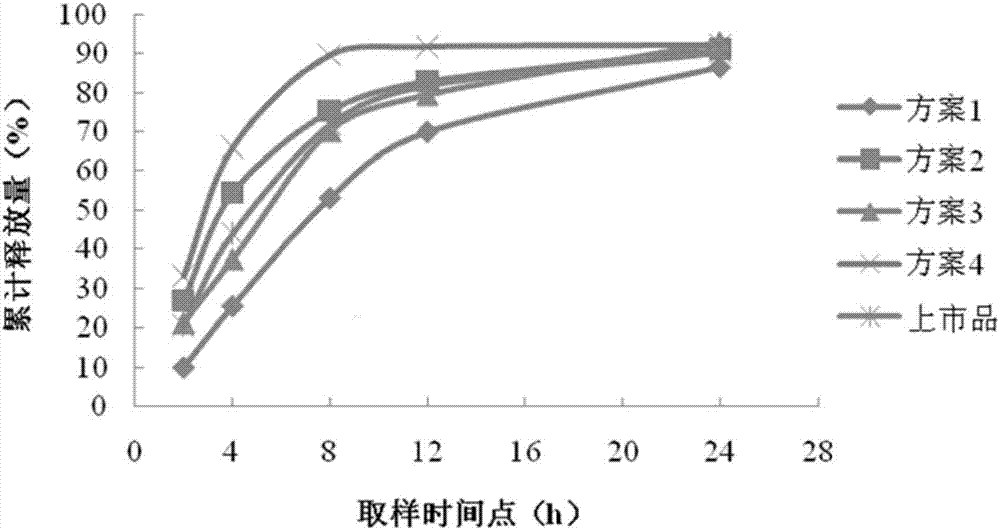
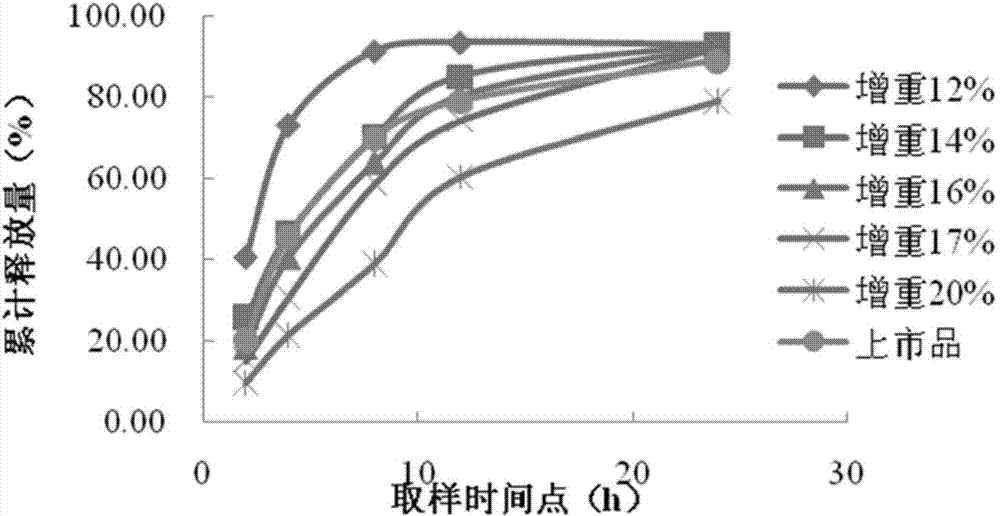
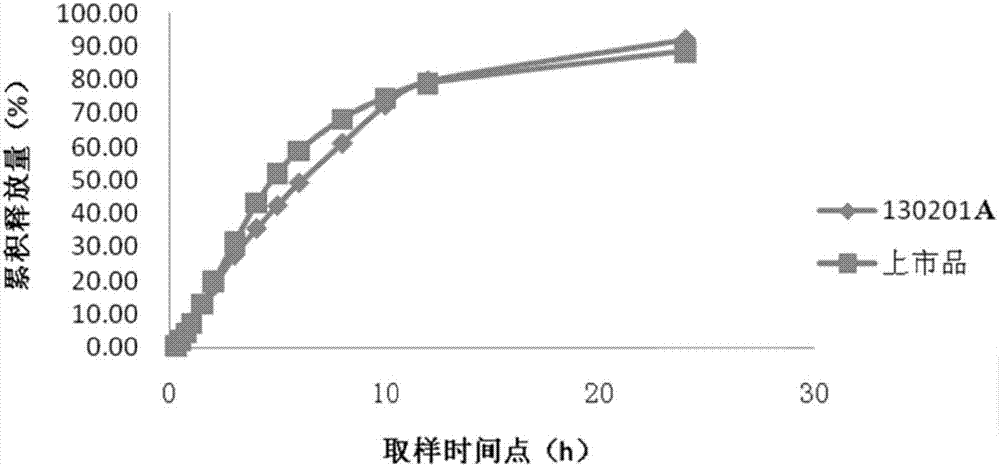
Venlafaxine hydrochloride sustained-release capsule composition
A technology of venlafaxine hydrochloride and sustained-release capsules, which is applied in the field of medicine, can solve problems such as adhesion and excessive softening of polymers, and achieve the effect of reducing side effects and avoiding adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] According to above-mentioned screening result, decide to adopt the prescription (specification: 75mg) as shown below to carry out the preparation of sustained-release capsule composition:
[0085]
[0086] The preparation method is as follows:
[0087] (1) Take by weighing 6660g of ethanol, place in a clean container, add 1273g of venlafaxine hydrochloride, stir to make the dissolution even, then slowly add 225g of hypromellose, vigorously stir to make the dispersion uniform, cross a 100 mesh sieve, and obtain Drug-loaded layer liquid;
[0088] (2) Put 1650g of microcrystalline cellulose pellet cores in a fluidized bed, set the air inlet temperature to 39°C, set the frequency, and after drying for 30 minutes, set a certain atomization pressure and needle pressure, and adjust The height of the guide tube is to make the core of the microcrystalline cellulose pellets fluidized evenly. After controlling the temperature of the material to 33-37°C, start to spray the core...
Embodiment 2
[0093] According to above-mentioned screening result, decide to adopt the prescription (specification: 150mg) as shown below to carry out the preparation of sustained-release capsule composition:
[0094]
[0095]
[0096] The preparation method is as follows:
[0097] (1) Take by weighing 6660g of ethanol, place in a clean container, add 1273g of venlafaxine hydrochloride, stir to make the dissolution even, then slowly add 225g of hypromellose, vigorously stir to make the dispersion uniform, cross a 100 mesh sieve, and obtain Drug-loaded layer liquid;
[0098] (2) Put 1650g of microcrystalline cellulose pellet cores in a fluidized bed, set the air inlet temperature to 39°C, set the frequency, and after drying for 30 minutes, set a certain atomization pressure and needle pressure, and adjust The height of the guide tube is to make the core of the microcrystalline cellulose pellets fluidized evenly. After controlling the temperature of the material to 33-37°C, start to spr...
Embodiment 3
[0103] According to above-mentioned screening result, decide to adopt the prescription (specification: 75mg) as shown below to carry out the preparation of sustained-release capsule composition:
[0104]
[0105] The preparation method is as follows:
[0106] (1) Take by weighing 9300g of ethanol, place in a clean container, add 1867g of venlafaxine hydrochloride, stir to make the dissolution even, then slowly add 320g hypromellose, vigorously stir to make the dispersion uniform, cross a 100 mesh sieve, and obtain Drug-loaded layer liquid;
[0107] (2) Put 2337g of microcrystalline cellulose pellet cores in a fluidized bed, set the air inlet temperature to 39°C, set the frequency, and after drying for 30 minutes, set a certain atomization pressure and needle pressure, and adjust The height of the guide tube is to make the core of the microcrystalline cellulose pellets fluidized evenly. After controlling the temperature of the material to 33-37°C, start to spray the core of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com