Microcapsule with extended release active pharmaceutical ingredient and preparation method thereof
An active pharmaceutical ingredient, prolonged release technology, applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. The effect of targeted drug delivery and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
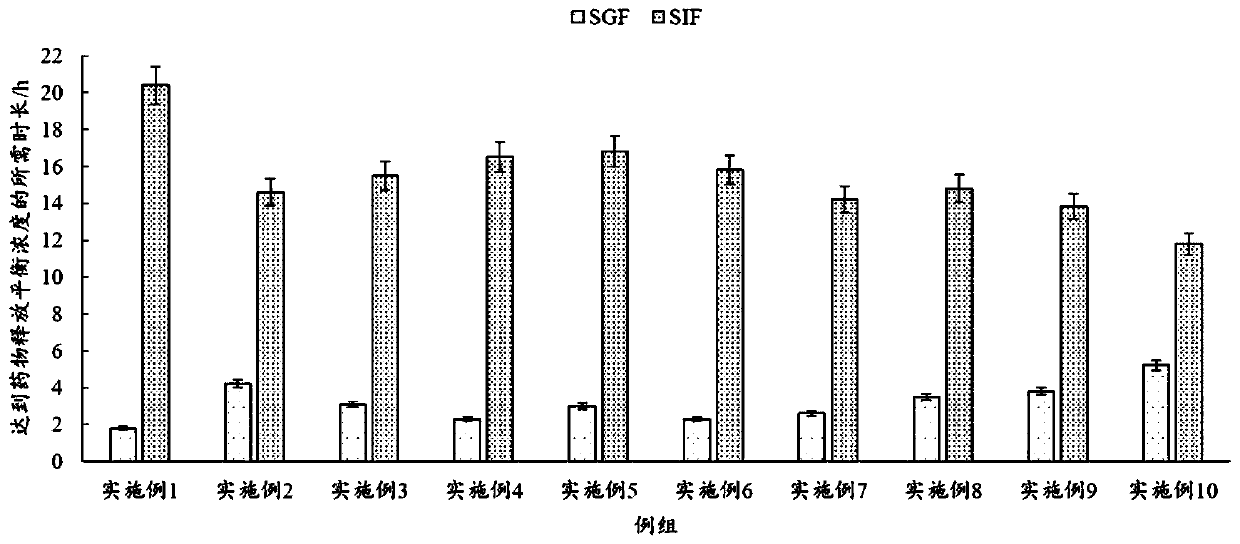
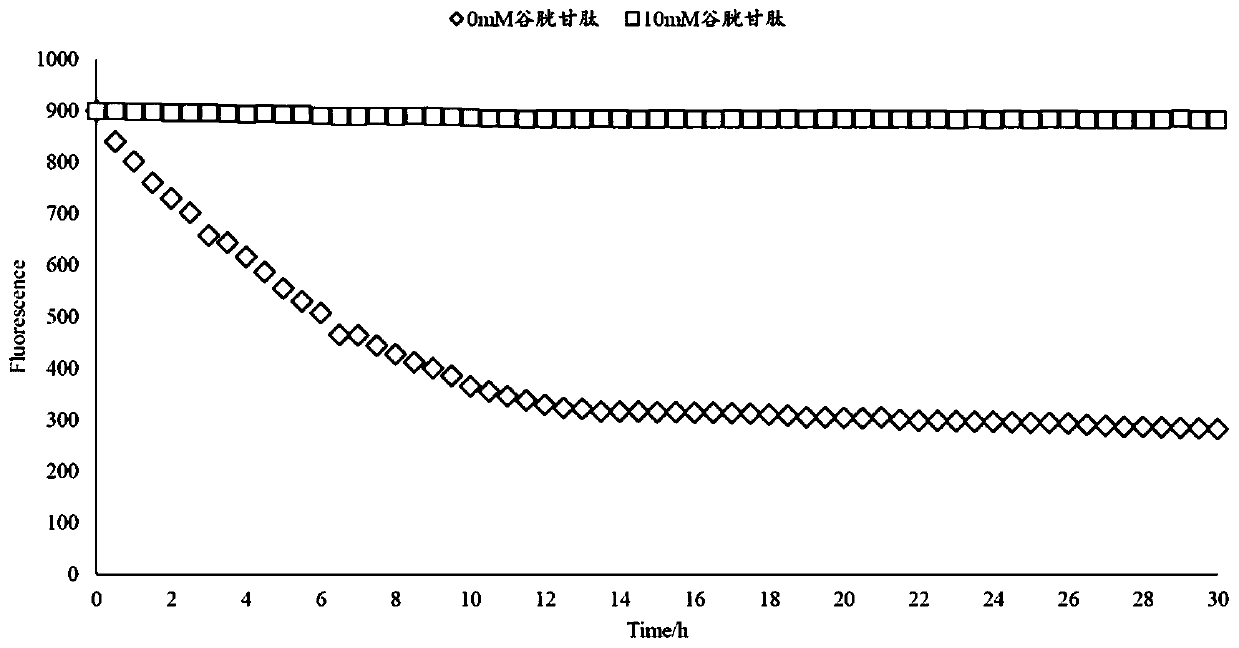
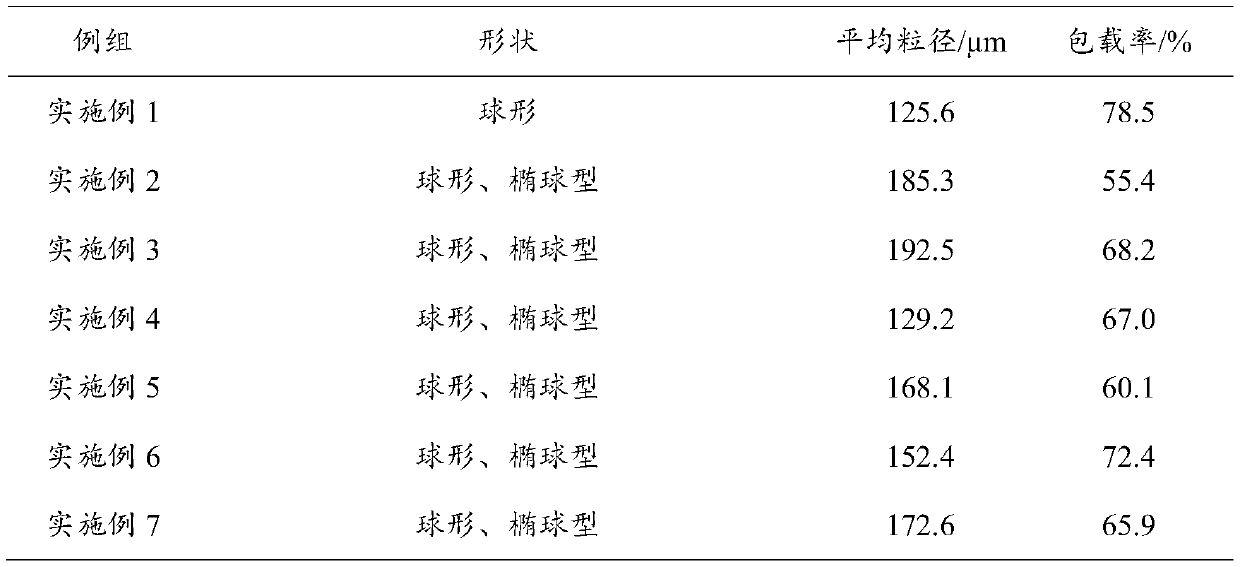
Image
Examples
Embodiment 1
[0066] : a microcapsule with extended release active pharmaceutical ingredient:
[0067] This embodiment provides a microcapsule with prolonged release of active pharmaceutical ingredients, which is prepared through the following steps:
[0068] 1) At a temperature of 50°C, the modified low-substituted arginine 9-mer / chitosan was dissolved in a large amount of 0.5wt% acetic acid solution, and then nano-active bamboo charcoal powder and levofloxacin hydrochloride solution were added successively, and fully mixed;
[0069] 2) Under heat preservation conditions, slowly drop 5.0 wt% sodium pyrophosphate solution into the mixed solution in step 1), filter out after crosslinking for 1 hour, wash the residual solution on the surface, rinse it with ethanol for 5 times, and dry it at room temperature.
[0070] In the present embodiment, the modified low-substituted arginine 9-mer / chitosan is prepared by the following steps:
[0071]1) Slowly add the tetrahydrofuran solution dissolved ...
Embodiment 2
[0085] : Another microcapsule with extended release active pharmaceutical ingredients:
[0086] This embodiment 2 provides another kind of drug-loaded microcapsules, the preparation method of which is basically the same as that of embodiment 1, except that in this embodiment the relative molecular mass is 5 Chitosan replaces the modified low-substituted arginine 9-mer / chitosan, and the rest of the components and steps are the same as in Example 1.
Embodiment 3
[0087] : Another microcapsule with extended release active pharmaceutical ingredients:
[0088] This embodiment 3 provides another kind of drug-loaded microcapsules, the preparation method of which is basically the same as that of embodiment 1, the only difference being that in this embodiment, the relative molecular mass of the arginine 9-mer with a weight ratio of 1:6.0 is 1.2×10 5 The chitosan was simply mixed without double-check modification to replace the modified low-substituted arginine 9-mer / chitosan complex, and the rest of the components and steps were the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com