Trimetazine dihydrochloride sustained-release tablet and preparation method thereof
A technology of trimetazidine hydrochloride and sustained-release tablets, which is applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problem of poor stability and easy degradation of trimetazidine hydrochloride tablets. and other problems, to achieve the effect of stable process, stable dissolution and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
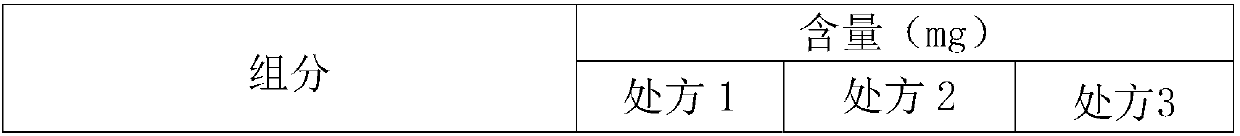
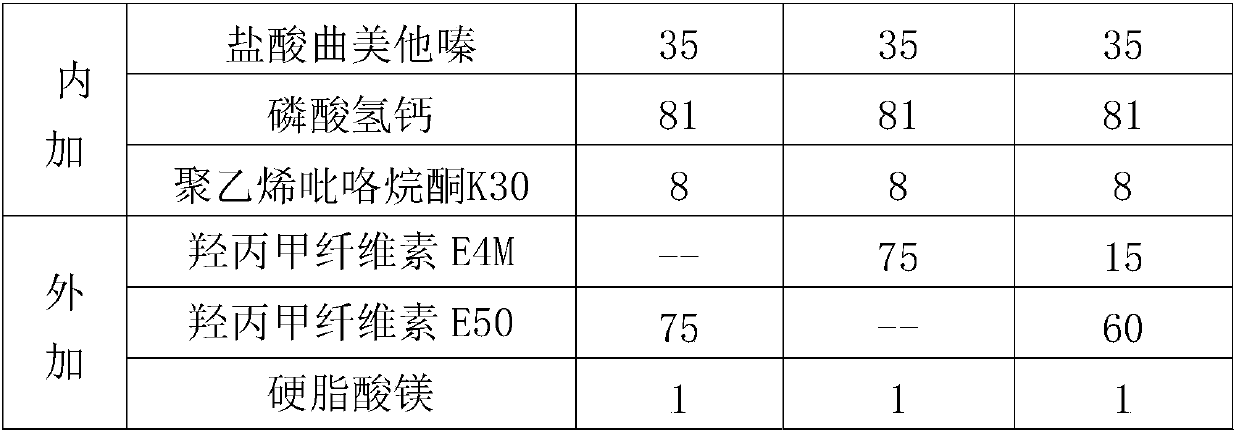
[0049] 1. Prescription
[0050]
[0051]
[0052] Preparation Process
[0053] (1) Mix the prescribed amount of trimetazidine hydrochloride, calcium hydrogen phosphate and polyvinylpyrrolidone evenly.
[0054] (2) Add appropriate amount of purified water for wet granulation and fluidized bed drying.
[0055] (3) Mix the granules prepared in step (2) with prescription quantities of hypromellose E50 and hypromellose E4M, add magnesium stearate and mix uniformly;
[0056] (4) Compressing the mixture obtained in step (3) into tablets and coating with non-functionality.
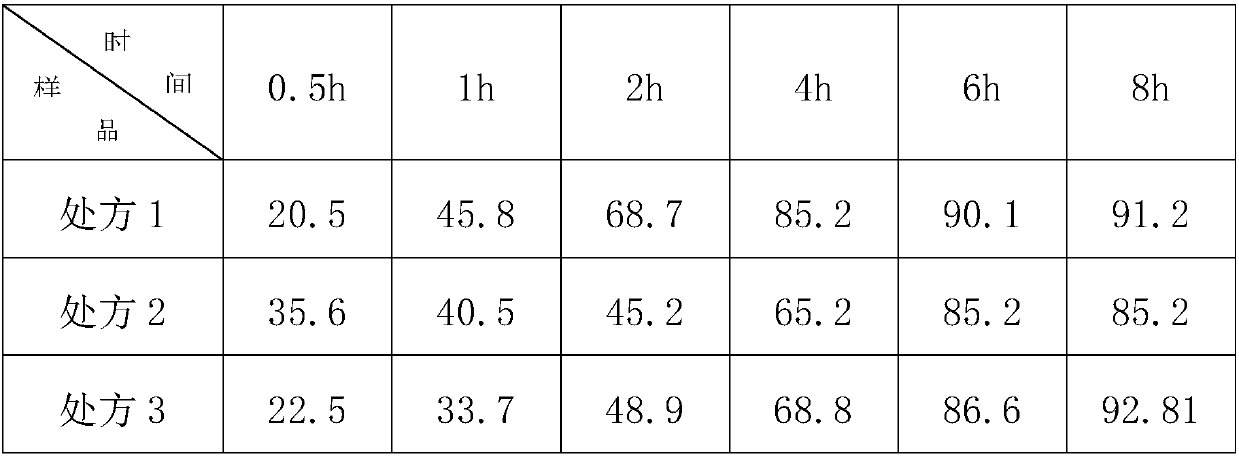
[0057] The sample that embodiment 1 obtains is put in the phosphate buffer saline solution of pH6.8 and measures release rate as shown in the table below:
[0058]
[0059] Investigating the release curve of the trimetazidine hydrochloride sustained-release tablets obtained in Example 1, it can be seen that using a mixture of hypromellose with different viscosities as the matrix material can better con...
Embodiment 2
[0061] prescription:
[0062]
[0063]
[0064] Preparation Process:
[0065] (1) Mix the prescribed amount of trimetazidine hydrochloride, calcium hydrogen phosphate and polyvinylpyrrolidone evenly.
[0066] (2) Add appropriate amount of purified water for wet granulation and fluidized bed drying.
[0067] (3) Mix the granules prepared in step (2) with prescription quantities of hypromellose E50 and hypromellose E4M, add magnesium stearate and mix uniformly;
[0068] (4) Compressing the mixture obtained in step (3) into tablets and coating with non-functionality.
[0069] The sample that embodiment 2 is obtained is put in the phosphate buffer saline buffer solution of pH6.8 and measures release rate and is:
[0070]
[0071] Investigating the release curves of the trimetazidine hydrochloride sustained-release tablets obtained in Example 2, it can be seen that different types of binders have little effect on the release of trimetazidine hydrochloride sustained-releas...
Embodiment 3
[0076] 1. Prescription
[0077]
[0078] Preparation Process
[0079] (1) Mix the prescription amount of trimetazidine hydrochloride, calcium hydrogen phosphate and binder evenly.
[0080] (2) Add appropriate amount of purified water for wet granulation and fluidized bed drying.
[0081] (3) Mix the granules prepared in step (2) with prescription quantities of hypromellose E50 and hypromellose E4M, add magnesium stearate and mix uniformly;
[0082] (4) Compressing the mixture obtained in step (3) into tablets and coating with non-functionality.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com