GLP-1 analogue composition for microneedle devices
A technology of GLP-1 and analogs, which is applied in the field of GLP-1 analog compositions for microneedle devices, can solve the problems of increased burden on patients, and achieve the effects of suppressing liquid dripping, good release, and high viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6
[0081] In a test tube, lixisenatide and a solvent were mixed at the content ratio shown in Table 1. The prepared mixed solution was stirred with a stirrer (1500 rpm, 12 hours, 25° C.), and the solubility of the polymer compound was visually observed and evaluated according to the following criteria. The evaluation results are shown in Table 1.
[0082] A: completely dissolved
[0083] B: partially dissolved
[0084] C: not dissolved
[0085] Based on the feeling when mixing, the viscosity of the prepared mixture was evaluated according to the criteria described later.
[0086] a: suitable for coating
[0087] b: not suitable for coating
[0088] [Table 1]
[0089]
[0090] Macrogol 200 and macrogol 400 in the table are polyethylene glycols with molecular weights of 200 and 400, respectively.
Embodiment 7
[0092] The solvent is water, and the polymer carrier is added, and mixed with the content ratio shown in Table 2. Compatibility and viscosity of the mixture were evaluated in the same manner as in Examples 1-6. The evaluation results are shown in Table 2.
[0093] [Table 2]
[0094]
[0095]
[0096] PEG400 and PEG4000 in the table are polyethylene glycol with a weight average molecular weight of 400 and 4,000 respectively, D×40 and D×70 are dextran with a weight average molecular weight of 40,000 and 70,000 respectively, and HPC─M is a weight average molecular weight of 110,000-150,000 hydroxypropyl cellulose, CMC (250,000) is sodium carboxymethyl cellulose with a weight average molecular weight of 250,000.
[0097] Examples 1-6 showed excellent solubility and viscosity. Example 7 also shows excellent solubility and viscosity. In Example 7, no viscosity-increasing effect was observed by adding the polymer carrier, but it is believed that the viscosity-increasing eff...
Embodiment 8~10
[0100] In a test tube, lixisenatide and a solvent (and a polymer carrier) were mixed at the mass ratio shown in Table 3 to obtain a GLP-1 analog composition. Example 8 is a water formula composition using water as a solvent, Example 9 is a PG formula composition using propylene glycol (PG) as a solvent, and Example 10 is a G formula composition using glycerin (G) as a solvent.
[0101] [table 3]
[0102]
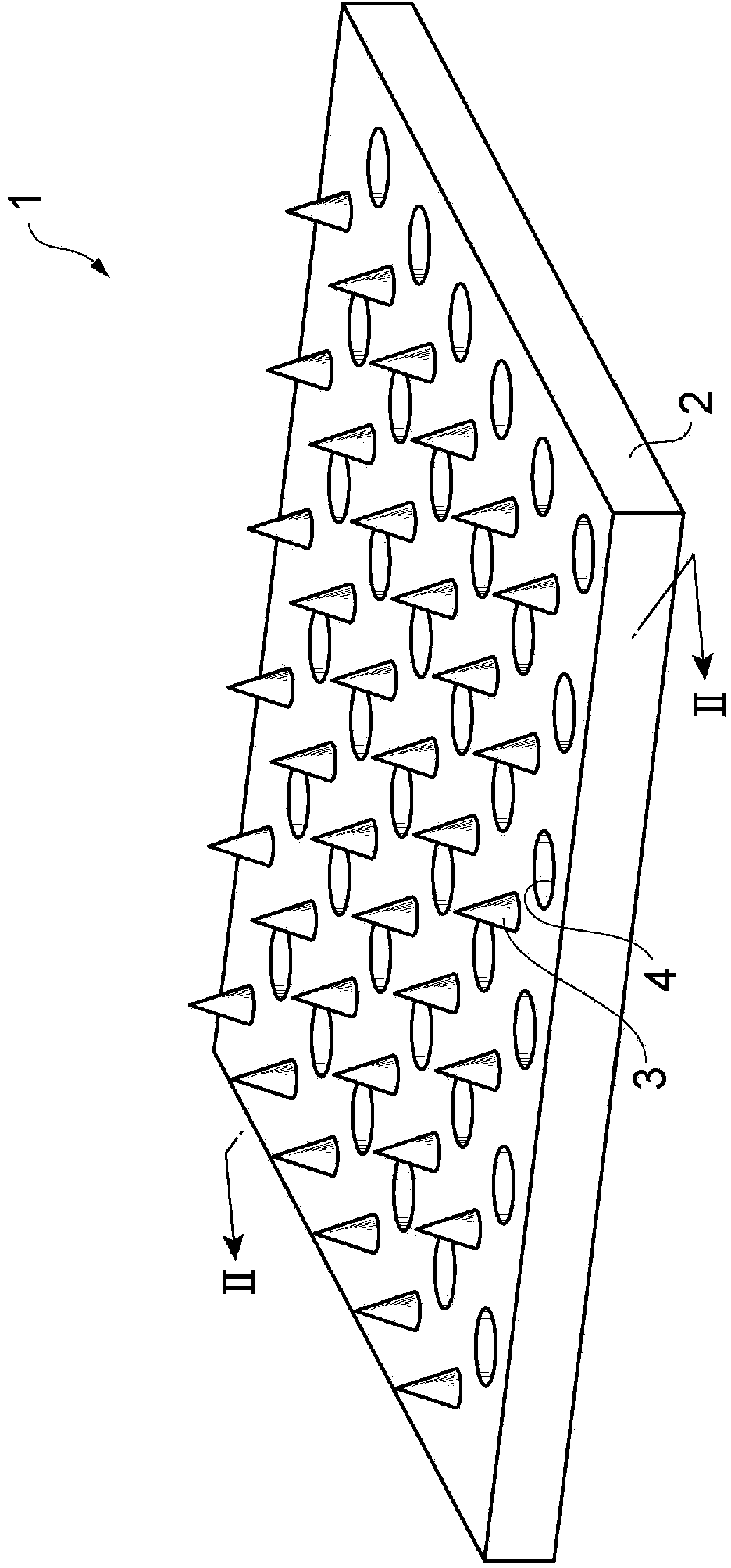
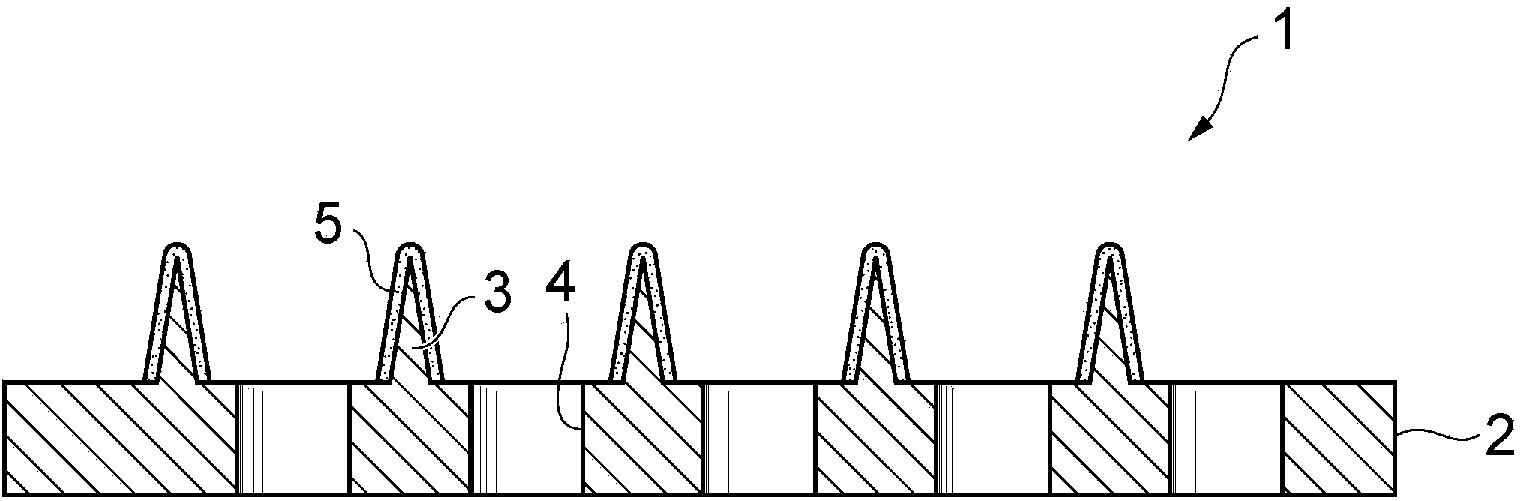
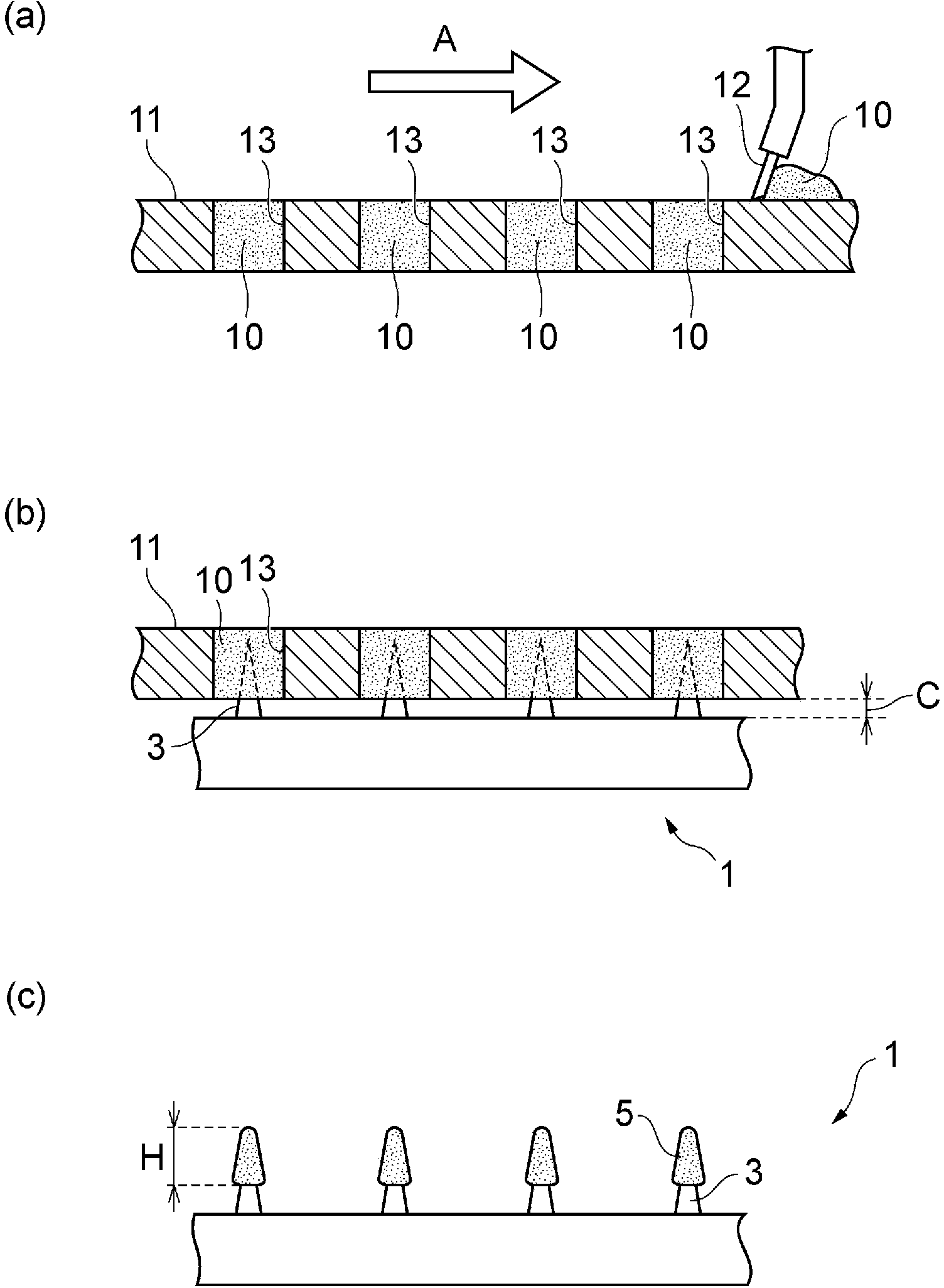
[0103] The aforementioned GLP-1 analog composition was coated on the microneedles from the needle tip to a height of 100 μm to obtain a coated microneedle array.
[0104] In Example 9 of the PG formulation and Example 10 of the G formulation, in addition to the high viscosity of the solvent itself, plus the viscosity from lixisenatide, a GLP-1 analog combination with a good viscosity of about 10,000 cps can be prepared things. On the other hand, in Example 8, a GLP-1 analog composition with good viscosity was prepared by the effect of pullulan as a viscosity-imparting a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com