Liraglutide multivesicular liposome and preparation method and application thereof
A polyvesicular liposome and liraglutide technology, which is applied in the field of medicine, can solve the problems of denaturation, inflammatory response, and activity decline of polypeptide and protein drugs, and achieve stable drug release rate, prolong circulation time, and high encapsulation. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
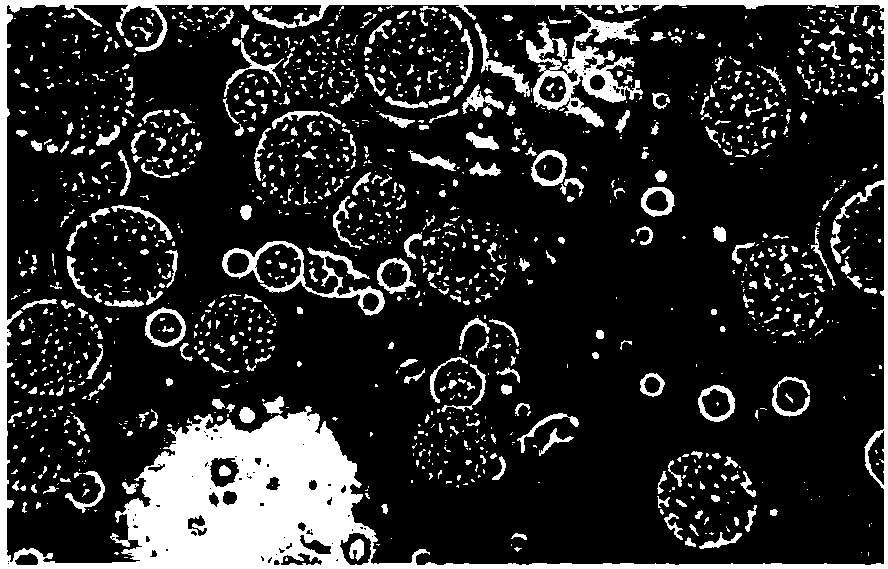
[0142]Precisely weigh 10.0mg of liraglutide and dissolve it in 2ml aqueous solution containing 0.8% human serum albumin and 7% sucrose to form an inner water phase; precisely weigh 201.1mg of soybean lecithin, 90.2mg of cholesterol, and 125.0mg of triolein , dissolved in 5ml ether to form an oil phase, add the above-mentioned internal water phase to the oil phase, and vortex for 3 minutes to form a W / O emulsion. Drop this emulsion into 7ml of 7% sucrose aqueous solution of 6mg / ml lysine, and keep stirring at 40°C for 5 minutes to form a W / O / W type double emulsion. Afterwards, it was moved to a rotary evaporator to remove residual organic solvents, and the liraglutide sustained-release multivesicular liposomes were obtained. The measured encapsulation efficiency was 87.5%, the drug loading was 2.3%, and the average particle size was 12.7 μm ( figure 1 ).
Embodiment 2
[0144] Accurately weigh 10.0mg of liraglutide and dissolve it in 2ml of 1% human serum albumin and 7% sucrose aqueous solution to form an inner water phase; accurately weigh 203.0mg of hydrogenated soybean lecithin, 96.6mg of cholesterol, triolein 121.0mg, dissolved in 5ml of chloroform to form an oil phase, add the above internal water phase to the oil phase, vortex to form a W / O emulsion. Drop this emulsion into 7ml of 7% sucrose aqueous solution containing 6mg / ml lysine, and continue to stir at 40°C for 10 minutes to form a W / O / W type double emulsion. , move it to rotary evaporator to remove residual organic solvent to obtain liraglutide sustained-release multivesicular liposomes, the measured encapsulation efficiency is 74.4%, the drug loading is 2.3%, and the average particle diameter is 22.0 μm ( figure 2 ).
Embodiment 3
[0146] Accurately weigh 10.0mg of liraglutide and dissolve it in 2ml of aqueous solution containing 0.9% human serum albumin and 7% sucrose to form an inner water phase; accurately weigh 196.8mg of dioleoylphosphatidylcholine, 99.4mg of cholesterol, three oil Glyceride 126.1 mg, dissolved in 6 ml of chloroform to form an oil phase, the above-mentioned internal water phase was added to the oil phase, vortexed for 5 min to form a W / O emulsion. Under stirring at 1000r / min, drop the emulsion into 20ml of 8% sucrose aqueous solution containing 5mg / ml lysine, stir continuously at 40°C for 10min to form a W / O / W type double emulsion, and blow in nitrogen to make the organic solvent naturally Volatilize, and when sedimentation occurs, move it to a rotary evaporator to remove residual organic solvents to obtain liraglutide sustained-release multivesicular liposomes. The measured encapsulation efficiency is 88.0%, and the drug loading is 2.4%. The particle size is 11.6 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com