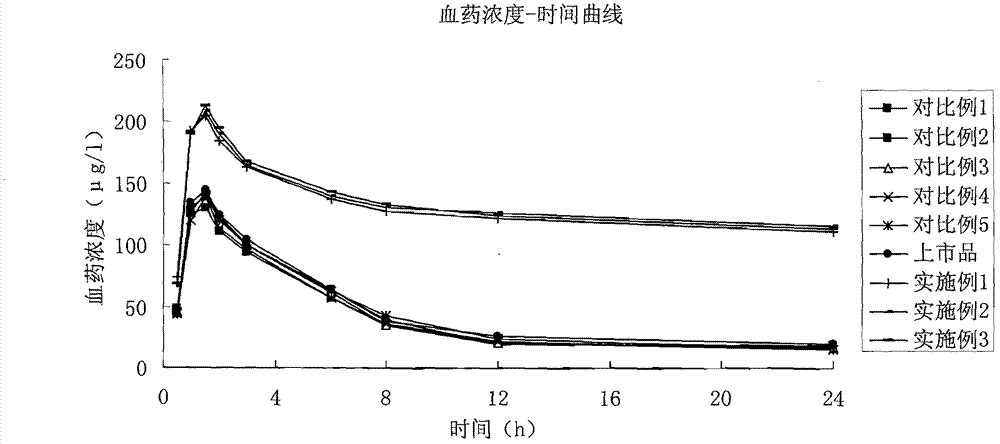
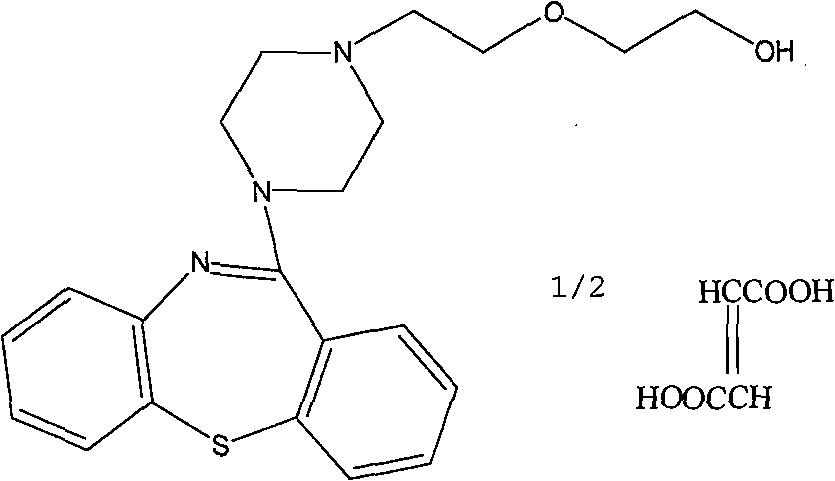
Solid quetiapine fumarate liposome preparation
A technology for quetiapine fumarate and solid preparations, applied in the field of pharmaceutical preparations, can solve problems such as unfavorable long-term storage, unsatisfactory drug stability, hidden dangers in clinical use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of quetiapine fumarate liposome tablets
[0062] The raw and auxiliary materials used are as follows:
[0063]
[0064] Adopt following production process to prepare quetiapine fumarate liposome tablet:
[0065] (1) 200g egg yolk lecithin, 120g cholesterol, 80g soybean sterol, 50g sorbitan stearate are dissolved in 1500ml acetone solvent, mix uniformly, remove organic solvent under reduced pressure on rotary evaporator, make phospholipid film;
[0066] (2) add 1500ml pH and be the phosphate buffer solution of 7.0, shake, stir to make phospholipid membrane fully hydrate, 3000rpm homogeneous emulsification, microporous membrane filtration, make blank liposome suspension;
[0067] (3) 100g of quetiapine fumarate was dissolved in the blank liposome suspension, filtered through a 0.45 μm microporous membrane, incubated at 59° C. for 30-40 minutes, and spray-dried to obtain a liposome solid;
[0068] (4) Mix quetiapine fumarate liposome solid with ...
Embodiment 2
[0070] Example 2 Preparation of quetiapine fumarate liposome tablets
[0071] The raw and auxiliary materials used are as follows:
[0072]
[0073] Adopt following production process to prepare quetiapine fumarate liposome tablet:
[0074] (1) 100g egg yolk lecithin, 60g cholesterol, 30g soy sterol, 45g sorbitan stearate are dissolved in 800ml acetone solvent, mix uniformly, remove organic solvent under reduced pressure on the rotary evaporator, make phospholipid film;
[0075] (2) Add 800ml of phosphate buffer solution with a pH of 7.0, shake and stir to fully hydrate the phospholipid membrane, homogeneously emulsify at 3000rpm, and filter through a microporous membrane to obtain a blank liposome suspension;
[0076] (3) 50g of quetiapine fumarate was dissolved in blank liposome suspension, filtered through a 0.45 μm microporous membrane, incubated at 59° C. for 30-40 minutes, and spray-dried to obtain a liposome solid;
[0077] (4) Mix quetiapine fumarate liposome so...
Embodiment 3
[0079] Example 3 Preparation of quetiapine fumarate liposome tablets
[0080] The raw and auxiliary materials used are as follows:
[0081]
[0082] Adopt following production process to prepare quetiapine fumarate liposome tablet:
[0083] (1) 50g egg yolk lecithin, 30g cholesterol, 20g soy sterol, 12g sorbitan stearate are dissolved in 400ml acetone solvent, mix uniformly, remove organic solvent under reduced pressure on rotary evaporator, make phospholipid film;
[0084] (2) Add 400ml pH of phosphate buffered saline solution of 7.0, shake and stir to make the phospholipid film fully hydrated, homogeneously emulsify at 3000rpm, and filter through a microporous membrane to obtain a blank liposome suspension;
[0085] (3) 25g of quetiapine fumarate was dissolved in the blank liposome suspension, filtered through a 0.45 μm microporous membrane, kept at 59° C. for 30-40 minutes, and spray-dried to obtain a liposome solid;
[0086] (4) Mix quetiapine fumarate liposome soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com