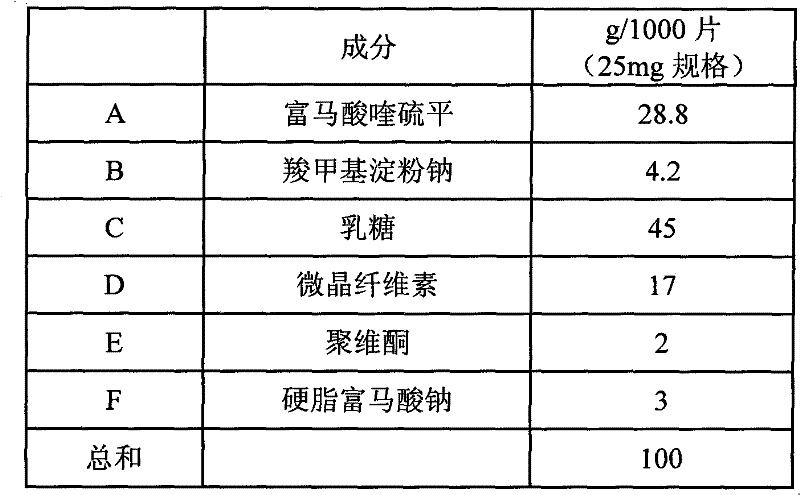
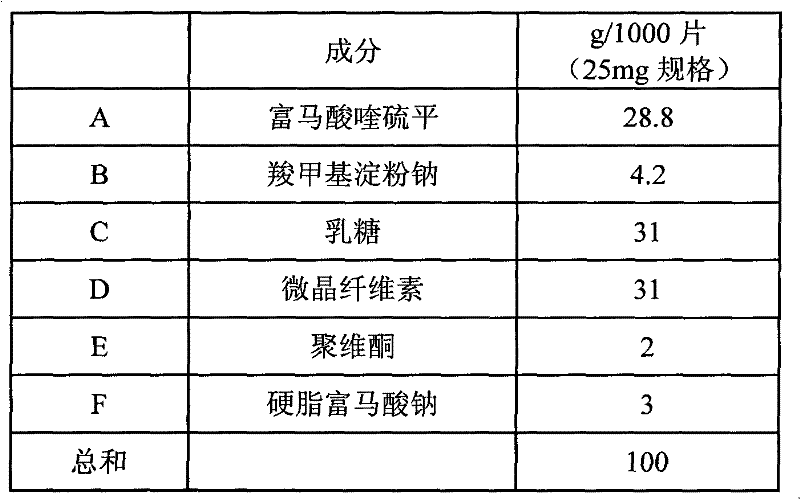
Crystalline quetiapine fumarate and pharmaceutical compositions thereof
A technology of quetiapine fumarate and crystalline form, which is applied in the direction of drug combination, pharmaceutical formula, carboxylate preparation, etc., which can solve the problems of unfavorable resource saving, large water consumption, and reduction of production costs, so as to overcome environmental pollution and high cost, the effect of significant cost and yield advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
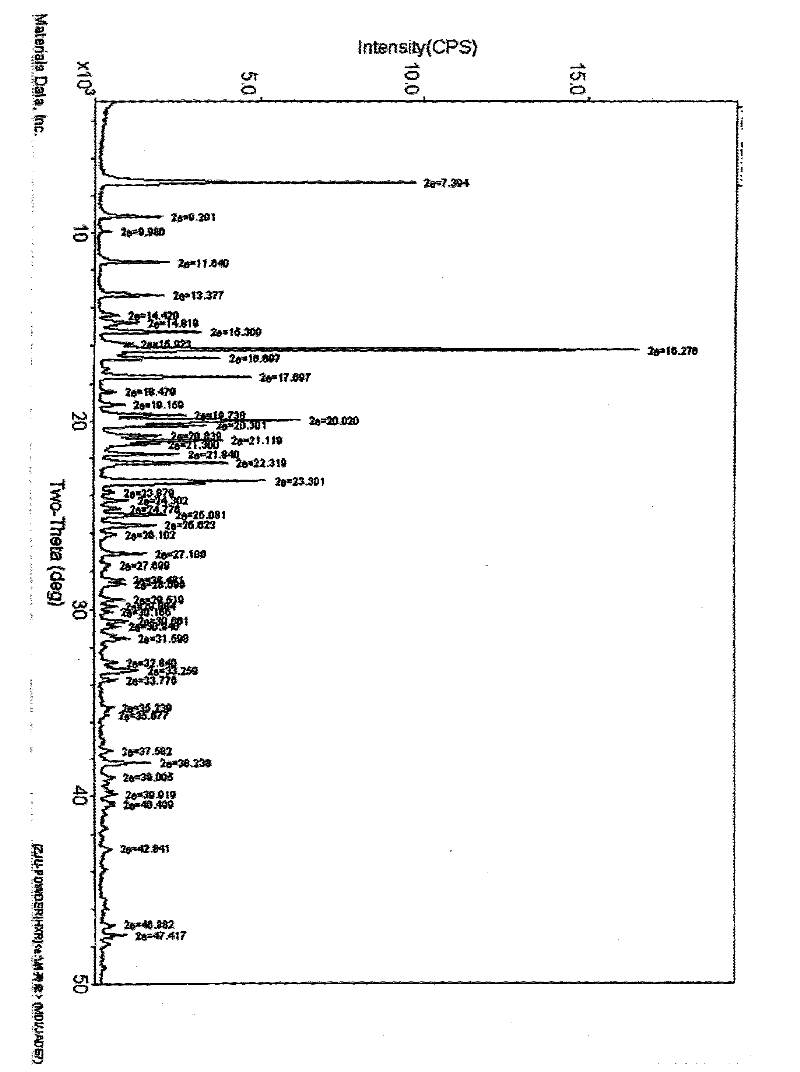
[0032] Take 5g of quetiapine fumarate, add 15g of water, stir and mix to obtain a mixture, heat to 95°C, the mixture becomes a clear solution, stir and cool, the solution is relatively viscous, mechanical stirring is required, quetiapine fumarate slowly precipitates, Filter under reduced pressure to obtain quetiapine fumarate crystals. After drying and weighing, the yield is 98%.
[0033] After HPLC analysis, the content after crystallization is 99.1%, and the related substances are <0.2%.
Embodiment 2
[0035] Take 5g of quetiapine fumarate, add 20g of water, stir and mix to obtain a mixture, heat to 92°C, the mixture becomes a clear solution. After stirring and cooling, quetiapine fumarate slowly precipitated, and filtered under reduced pressure to obtain quetiapine fumarate crystals. After drying and weighing, the yield is 93%.
[0036] After HPLC analysis, the content after crystallization is 99.6%, and the related substances are <0.1%. There is no change in related substances after crystallization.
Embodiment 3
[0038] Take 5g of quetiapine fumarate, add 30g of water, stir and mix to obtain a mixture, heat to 85°C, the mixture becomes a clear solution. After stirring and cooling, quetiapine fumarate slowly precipitated, and filtered under reduced pressure to obtain quetiapine fumarate crystals. After drying and weighing, the yield is 96%.
[0039] After HPLC analysis, the content after crystallization is 99.7%, and the related substances are <0.1%. There is no change in related substances after crystallization.
[0040] Through the above-mentioned examples, it is found that as the water consumption increases, the temperature required to obtain the quetiapine fumarate aqueous solution is lower, and if the water consumption is reduced, a higher temperature is required to obtain the quetiapine fumarate solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com