Sustained-release tablet containing quetiapine fumarate and preparation method of sustained-release tablet
A technology for quetiapine fumarate and sustained-release tablets, which is applied to medical preparations containing active ingredients, medical preparations with non-active ingredients, and pharmaceutical formulas, and can solve complex processes, high energy consumption, and increased production. cost and other issues, to achieve the effect of simple preparation process and lower production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0035] Below in conjunction with specific embodiment, further illustrate the present invention. It should be understood that these examples are only used to illustrate the present invention and are not intended to limit the scope of the present invention. All percentages, ratios, ratios, or parts are by weight unless otherwise indicated.
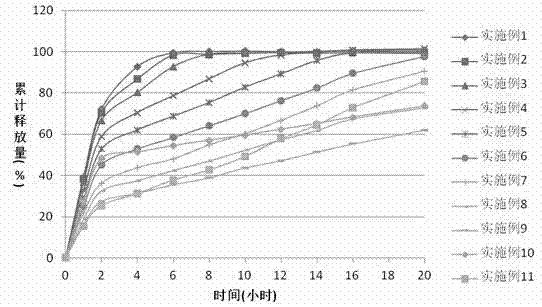
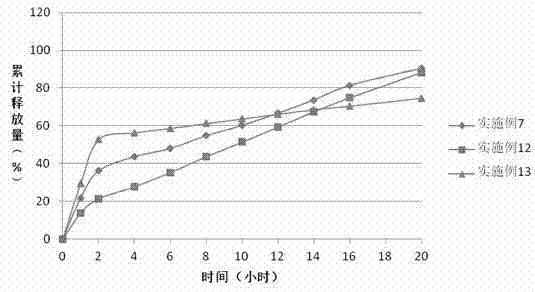
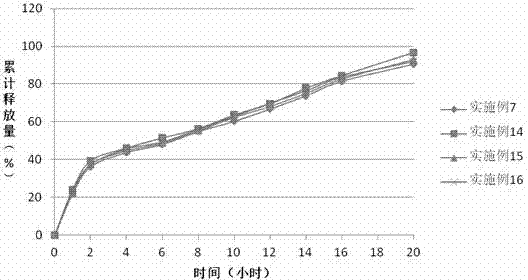
[0036]The sustained-release properties of the preparation in the examples of the present invention can be evaluated by in vitro dissolution methods. With reference to the first method of the second appendix X D of the Chinese Pharmacopoeia version in 2010, the device of the first method of the dissolution measurement method (Appendix X C) is adopted. 100 revolutions per minute, first use 750ml of hydrochloric acid solution (9→1000) as the solvent to release for 2 hours. Then add 250ml of 0.2mol / L sodium phosphate solution to the medium, adjust the pH to (6.8±0.05) with 2mol / ml hydrochloric acid solution or sodium hydroxide solution, and ope...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com