Preparation method of quetiapine fumarate
A technology of quetiapine fumarate and fumaric acid, which is applied in the field of synthesis and purification of quetiapine fumarate, can solve the problems of incomplete reaction of raw materials, low product purity, low yield, etc., and achieve the reduction of impurities , the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 2L three-necked flask, sequentially add 676g of n-propanol, 248g of N-methylpyrrolidone, 56g of polyethylene glycol 400, 11-piperazine-dibenzo[B,F][1,4]thiazepine disalt Salt 140g, anhydrous sodium carbonate 160g, potassium iodide 5g and 2-(2-chloroethoxy)ethanol 70g, reflux for 6h. Add 30 g of anhydrous sodium carbonate and 25 g of 2-(2-chloroethoxy)ethanol, and reflux for 4 hours. Add 20 g of anhydrous sodium carbonate and 15 g of 2-(2-chloroethoxy)ethanol, and reflux for 4 hours. The reaction solution is poured into 1.8 kg of ethyl acetate and washed with 2.5 kg of water. Dilute hydrochloric acid was added, and the reaction was stirred at room temperature for 1h. The aqueous phase was washed with 3.5 kg of dichloromethane and 2.2 kg of ethyl acetate, respectively. The water phase was adjusted to a pH value of 10-11 with saturated sodium carbonate aqueous solution, 2.7 kg of ethyl acetate was added to the water phase for extraction, the organic phase was washed...
Embodiment 2
[0026] Add 400g of ethyl acetate and 139g of crude quetiapine fumarate into a 1L three-neck flask, stir at room temperature for 2h, filter with suction, rinse with 200g of ethyl acetate, dry the filter cake at 50°C (blast oven) to constant weight, and obtain a white solid 131 g, yield: 94.2%.
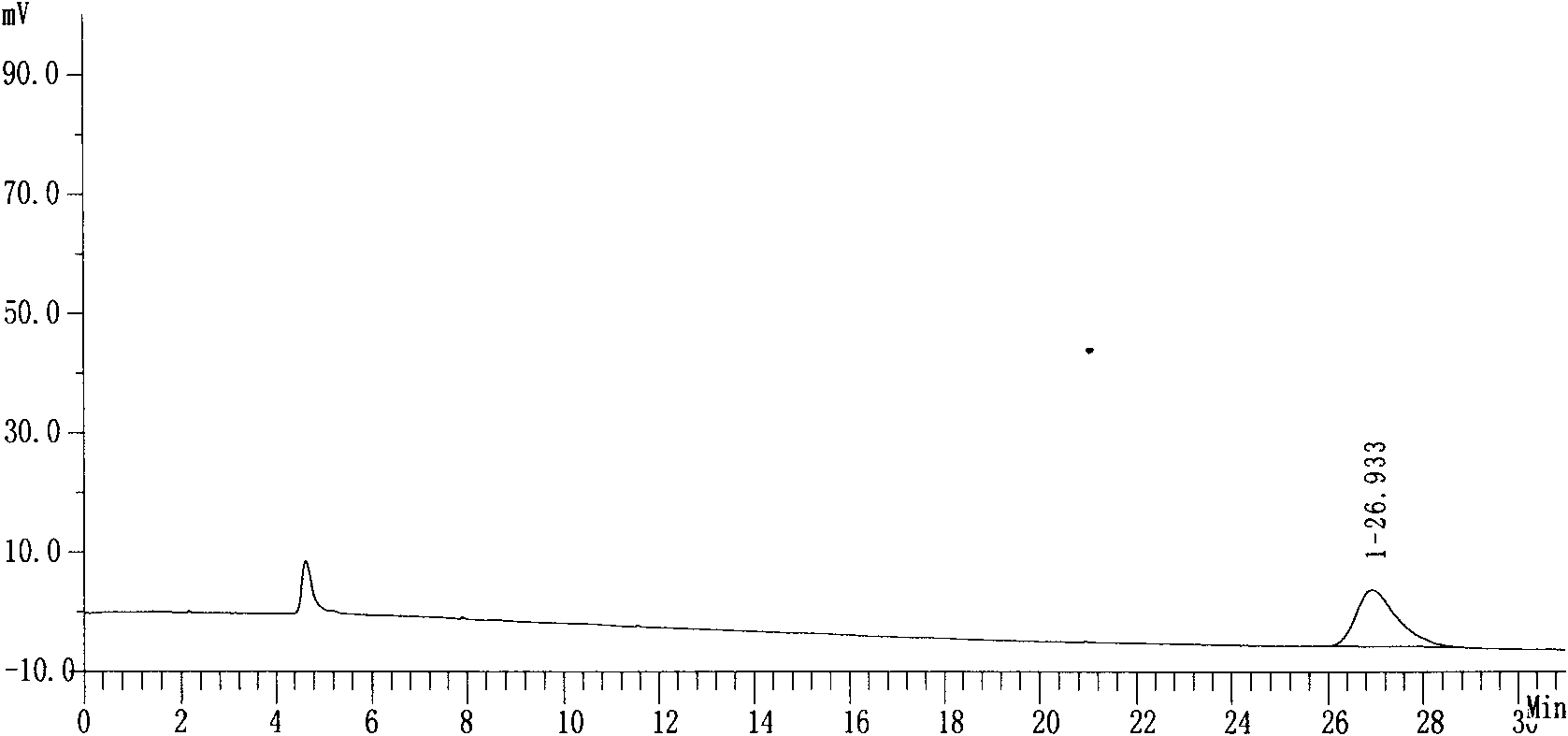
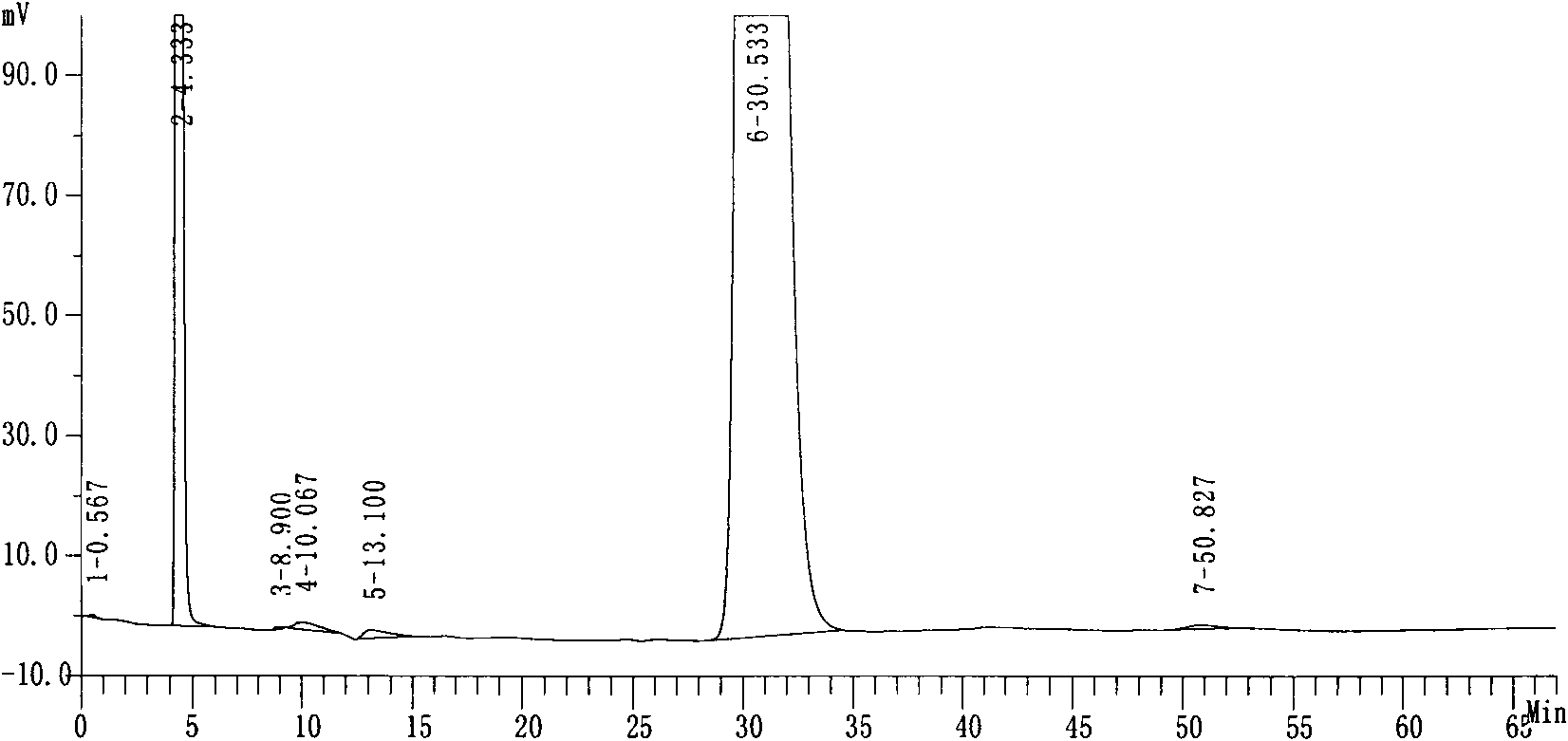
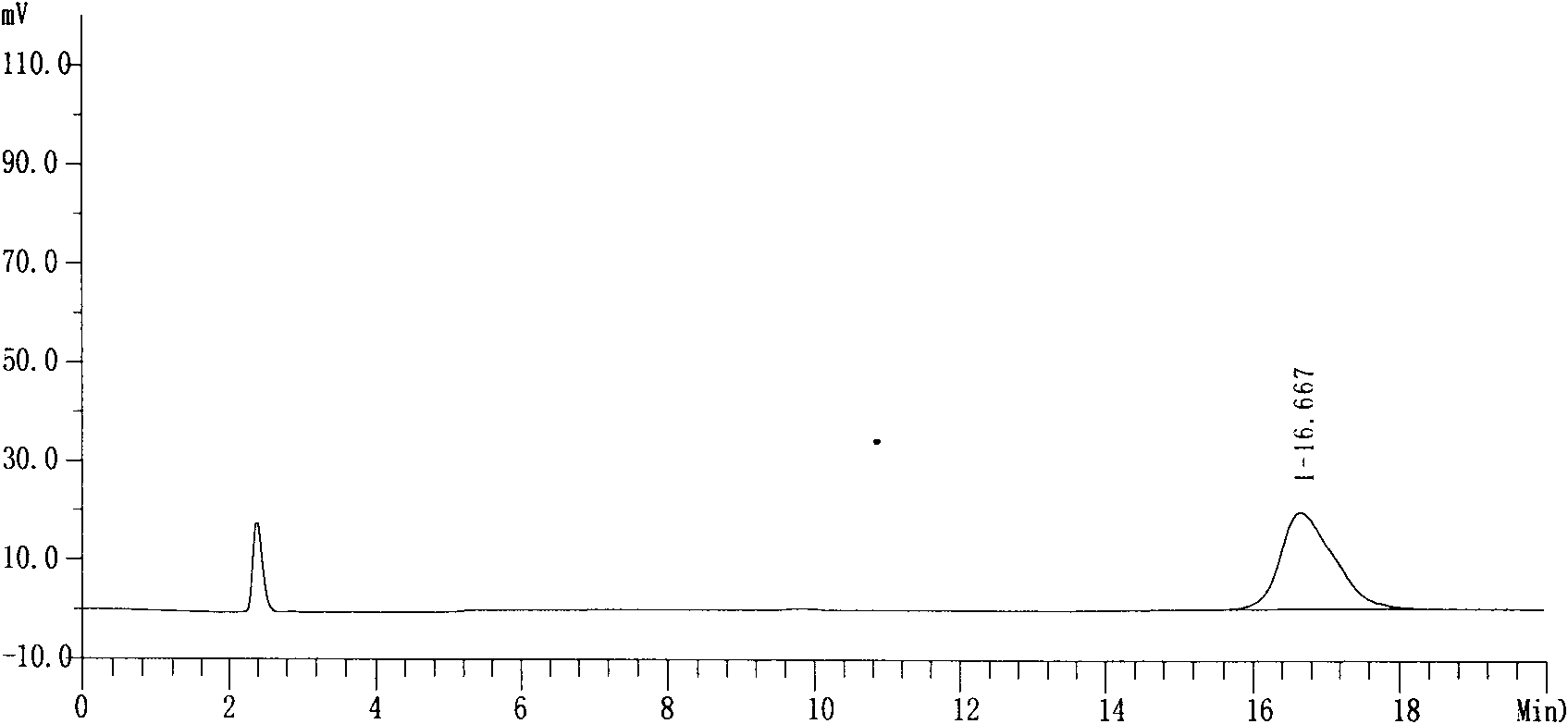
[0027] Purity: 99.74%, see attached data figure 1 ,2.
Embodiment 3
[0029] In a 5L reactor, sequentially add 1.69kg of n-propanol, 0.62kg of N-methylpyrrolidone, 140g of polyethylene glycol, 11-piperazine-dibenzo[B,F][1,4]thiazepine Hydrochloride 350g, anhydrous sodium carbonate 400g, potassium iodide 12.5g and 2-(2-chloroethoxy)ethanol 175g, reflux for 6h. Add 75g of anhydrous sodium carbonate and 62.5g of 2-(2-chloroethoxy)ethanol, and reflux for 4h. Add 50 g of anhydrous sodium carbonate and 32.5 g of 2-(2-chloroethoxy)ethanol, and reflux for 4 hours. The reaction solution is poured into 4.5 kg of ethyl acetate and washed with 6.25 kg of water. Dilute hydrochloric acid was added, and the reaction was stirred at room temperature for 1h. The aqueous phase was washed with 8.75 kg of dichloromethane and 5.5 kg of ethyl acetate, respectively. Adjust the pH value of the aqueous phase to 10-11 with saturated sodium carbonate aqueous solution, add 6.25kg of ethyl acetate to the aqueous phase for extraction, wash the organic phase with 2×5kg of wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com