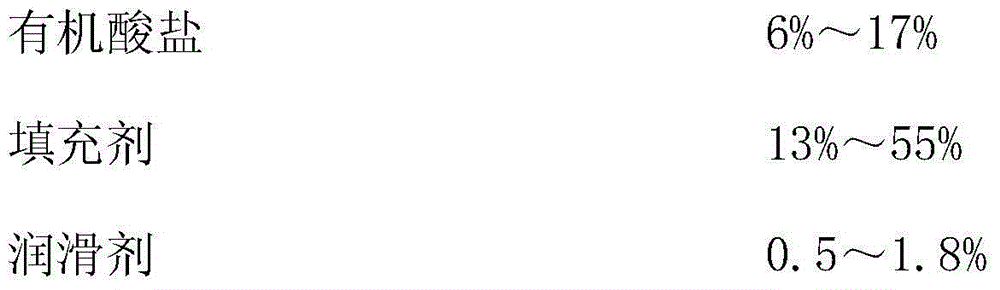Quetiapine fumarate sustained-release tablet and preparation method thereof
A technology for quinine fumarate and sustained-release tablets, which is applied in the field of drug sustained-release preparations and pharmaceutical preparations, can solve the problems of heavy drug tablets, many auxiliary materials, and unfavorable patient intake, and achieves simple preparation methods, good sustained-release performance, and large The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: Quetiapine fumarate sustained release tablet (10000 tablets)
[0038] components
weight%
Weight (Kg)
Quetiapine Fumarate
11.5%
0.575
[0039] Hydroxypropyl Methyl Cellulose (K4M)
7.6%
0.38
Hydroxypropyl Methyl Cellulose (K100M)
2.0%
0.10
Hydroxypropyl Methyl Cellulose (K100LV)
21.0%
1.05
sodium citrate
6.8%
0.34
25.4%
1.27
24.8%
1.24
1.0%
0.05
[0040]Among them, hydroxypropyl methylcellulose (K100M), hydroxypropyl methylcellulose (K4M) and hydroxypropyl methylcellulose (K100LV) are sustained-release materials, sodium citrate is an organic acid salt, lactose, micro Crystalline cellulose is used as a filler, and magnesium stearate is used as a lubricant.
[0041] Crushing the raw and auxiliary materials and passing through a 100-mesh sieve;
[0042] ...
Embodiment 2
[0043] Embodiment 2: Quetiapine fumarate sustained release tablet (10000 tablets)
[0044]
[0045]
[0046] Among them, hydroxypropyl methylcellulose (K100M), hydroxypropyl methylcellulose (K4M) and hydroxypropyl methylcellulose (K100LV) are sustained-release materials, sodium citrate is an organic acid salt, lactose, micro Crystalline cellulose is used as a filler, and magnesium stearate is used as a lubricant.
[0047] Crushing the raw and auxiliary materials and passing through a 100-mesh sieve;
[0048] According to the amount of 10,000 tablets, weigh quetiapine fumarate, organic acid salts, sustained-release materials and other pharmaceutical excipients except wetting agents and lubricants according to the formula ratio, mix them and place them in high-efficiency wet mixing granulation In the machine (GHL-30), add 2.5kg of wetting agent water to granulate, place it in a boiling granulator, adjust the inlet air temperature to 60°C and take 18 minutes to dry until t...
Embodiment 3
[0049] Embodiment 3: Quetiapine fumarate sustained release tablet (10000 tablets)
[0050]
[0051]
[0052] Among them, hydroxypropyl methylcellulose (K100M), hydroxypropyl methylcellulose (K4M) and hydroxypropyl methylcellulose (K100LV) are sustained-release materials, sodium citrate is an organic acid salt, lactose, micro Crystalline cellulose is used as a filler, and magnesium stearate is used as a lubricant.
[0053] Crushing the raw and auxiliary materials and passing through a 100-mesh sieve;
[0054] According to the amount of 10,000 tablets, quetiapine fumarate, organic acid salts, sustained-release materials and other pharmaceutical excipients except wetting agents and lubricants were weighed according to the formula ratio, mixed according to the formula amount, and placed in a high-efficiency wet method In the mixing granulator (GHL-30), add 2.5kg of wetting agent water to granulate, place it in a boiling granulator, adjust the inlet air temperature to 60°C a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com