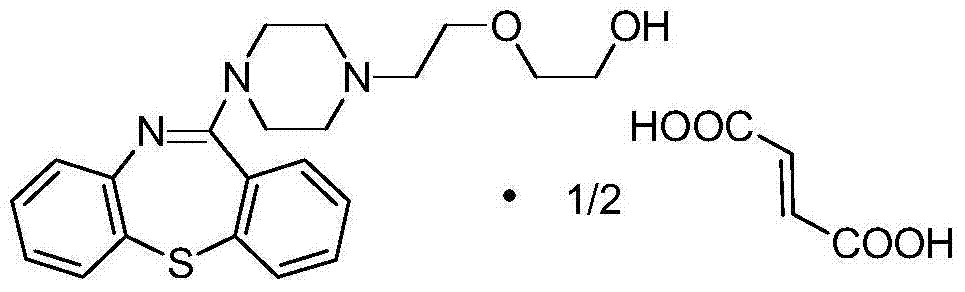
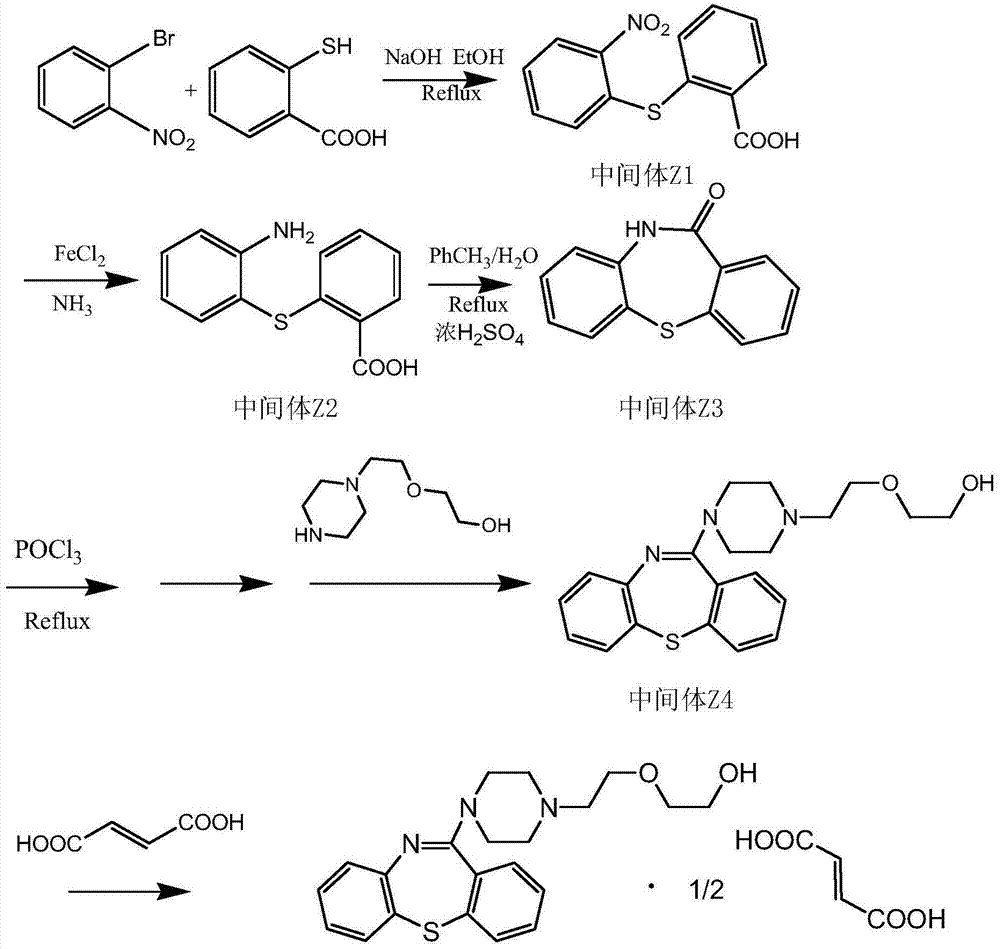
Synthetic method of thiazepine compound
A condensation and salt-forming technology, which is applied in the field of preparation of atypical antipsychotics, can solve problems such as severe operating conditions, unstable intermediates, and many impurities in the product, and achieve simple and safe operation, eliminate troublesome handling, and reduce the amount of three wastes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Preparation of intermediate Z1 (2-nitro-2'-carboxydiphenylsulfide):
[0019] Add 300ml of absolute ethanol to the reaction flask, add o-bromonitrobenzene (20g, 0.1mol) and thiosalicylic acid (15.4g, 0.1mol) while stirring, add sodium hydroxide (12g, 0.3mol) while stirring , slowly heated to reflux, reacted for 5 hours, cooled to room temperature, stirred for 2 hours, and filtered to obtain yellow solid intermediate Z1 (25.6g, yield 93.0%, mp164~165°C).
Embodiment 2
[0020] Embodiment 2: Preparation of intermediate Z2 (2-amino-2'-carboxydiphenylsulfide):
[0021] Add 200ml of purified water into the reaction flask, add intermediate Z1 (10g, 0.03mol) under stirring, add 150ml of ammonia water dropwise, stir for 15-20 minutes, add ferrous sulfate (15g, 0.1mol), stir and heat to reflux, and react After 6 hours, cool the reaction solution to 40-42°C, add 60ml of methanol, keep stirring for 20 minutes, continue to cool to 10-15 degrees, stir and crystallize for 6 hours, filter, wash the filter cake with a large amount of water, and dry to obtain an off-white solid Intermediate Z2 (6.5g, yield 88%, mp157-158°C).
Embodiment 3
[0022] Example 3: Preparation of intermediate Z3 (10H-dibenzo[b,f][1,4]thiazepin-11-one):
[0023] Add the mixed solvent of toluene / water (90ml / 10ml) into the reaction flask, add the intermediate Z2 (10g, 0.04mol) under stirring and slowly raise the temperature to 90-100 degrees, add concentrated sulfuric acid dropwise, after the addition is complete, raise the temperature to reflux, React for 4 to 5 hours until the Z2 content is less than 1%, slowly cool to room temperature, filter, soak the filter cake with a small amount of methanol, drain it, and dry it to obtain an off-white solid intermediate Z3 (8.5g, yield 92%, mp259~260℃).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com