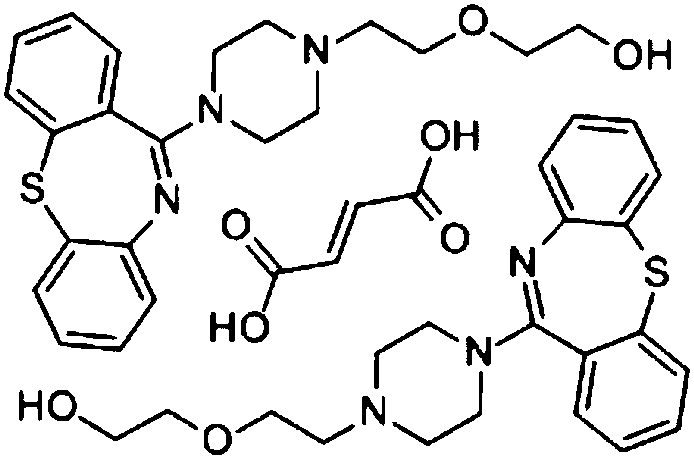
Quetiapine fumarate sustained-release tablet and preparation method thereof
A technology of quetiapine fumarate and quinolone fumarate, which is applied in the field of quetiapine fumarate sustained-release tablets and its preparation, can solve the problems of increased particle hardness, affecting sample dissolution, high viscosity, etc., to reduce contact time, easy control, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
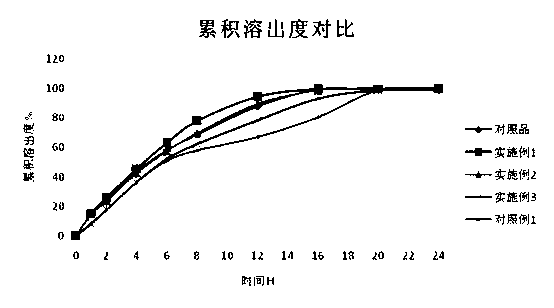
Image
Examples
Embodiment 1
[0020] Embodiment 1, quetiapine fumarate 40.15%, hypromellose 25.0%, lactose 10.50%, microcrystalline cellulose 17.00%, sodium citrate 6.35%, magnesium stearate 1.00%. Prepare as follows:
[0021] In the first step, quetiapine fumarate is crushed through a 100-mesh sieve, and hypromellose, lactose, microcrystalline cellulose, and sodium citrate are crushed through a 60-mesh sieve;
[0022] The second step weighs the first step of the prescription amount and sieves the raw and auxiliary materials, and mixes them evenly;
[0023] The third step and the second step mix the mixture evenly through a fluidized bed one-step granulator, the fan frequency is 10-13Hz, the spray frequency is 10Hz, the spray pressure is 0.15MPa, the material temperature is 30-60°C, and the air inlet temperature is 50-70 ℃, granulate with 50% ethanol aqueous solution as wetting agent;
[0024] In the fourth step, the fan frequency is 10-13Hz, the material temperature is 30-60°C, the air inlet temperature...
Embodiment 2
[0027] Example 2, quetiapine fumarate 40.15%, hypromellose 25.0%, lactose 10.50%, microcrystalline cellulose 17.00%, sodium citrate 6.35%, magnesium stearate 1.00%. Prepare as follows:
[0028] In the first step, quetiapine fumarate is crushed through a 100-mesh sieve, and hypromellose, lactose, microcrystalline cellulose, and sodium citrate are crushed through a 60-mesh sieve;
[0029] The second step weighs the first step of the prescription amount and sieves the raw and auxiliary materials, and mixes them evenly;
[0030] The third step and the second step mix the mixture evenly through a fluidized bed one-step granulator, the fan frequency is 10-13Hz, the spray frequency is 10Hz, the spray pressure is 0.15MPa, the material temperature is 30-60°C, and the air inlet temperature is 50-70 ℃, granulate with 40% ethanol aqueous solution as wetting agent;
[0031] In the fourth step, the fan frequency is 10-13Hz, the material temperature is 30-60°C, the air inlet temperature is...
Embodiment 3
[0034] Example 3, quetiapine fumarate 40.15%, hypromellose 25.0%, lactose 10.50%, microcrystalline cellulose 17.00%, sodium citrate 6.35%, magnesium stearate 1.00%. Prepare as follows:
[0035] In the first step, quetiapine fumarate is pulverized through a 100-mesh sieve, and hypromellose, lactose, microcrystalline cellulose, and sodium citrate are pulverized through a 60-mesh sieve;
[0036] The second step weighs the first step of the prescription amount and sieves the raw and auxiliary materials, and mixes them evenly;
[0037] The third step and the second step mix the mixture evenly through a fluidized bed one-step granulator, the fan frequency is 10-13Hz, the spray frequency is 10Hz, the spray pressure is 0.15MPa, the material temperature is 30-60°C, and the air inlet temperature is 50-70 ℃, granulate with 30% ethanol aqueous solution as wetting agent;
[0038] In the fourth step, the fan frequency is 10-13Hz, the material temperature is 30-60°C, the air inlet temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com