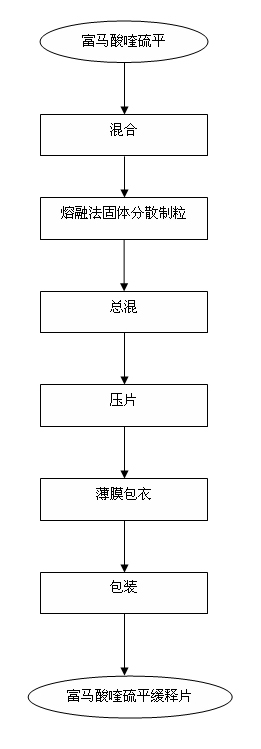
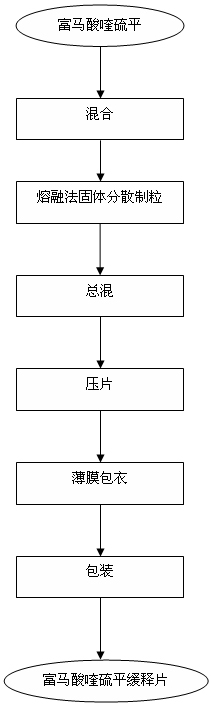
Preparation process of quetiapine fumarate slow release tablet
A technology of quetiapine fumarate and quinoline fumarate, which can be used in drug delivery, oil/fat/wax inactive ingredients, nervous system diseases, etc., and can solve the problems that the sustained-release tablet technology has not been reported in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Quetiapine Fumarate 50 mg, 200mg, 300mg, 400mg Sustained Release Tablets
[0035] prescription
quantity
content%
Quetiapine Fumarate
230.4 mg mg
46.08%
Kollicoat MAE 100P
69.6mg
13.92%
25mg
5.00%
hydrogenated castor oil
50mg
10.00%
110mg
22.00%
Micropowder silica gel
10mg
2.00%
5mg
1.00%
Total
500mg
100%
[0036] 1) Pass quetiapine fumarate through a 100-mesh sieve, pass hydrogenated castor oil through a 100-mesh sieve, add Sichuan wax powder and Kollicoat MAE 100P into a three-dimensional mixer and mix for 15 minutes, and spread the mixture evenly on a stainless steel plate superior.
[0037] 2) Put the stainless steel plate from operation 1) into an oven and heat it to 100°C, continue heating for 2 hours, take out the stainless steel plate from the oven and cool it down to r...
Embodiment 2
[0046] Preparation of Quetiapine Fumarate 50 mg, 200mg, 300mg, 400mg Sustained Release Tablets
[0047] prescription
quantity
content%
230.4mg
41.89%
Kollicoat MAE 100P
69.6mg
12.65%
30mg
5.46%
hydrogenated castor oil
55mg
10.00%
145mg
26.36%
Micropowder silica gel
15mg
2.73%
5mg
0.91%
Total
550mg
100%
[0048] 1) Pass quetiapine fumarate through a 100-mesh sieve, pass hydrogenated castor oil through a 100-mesh sieve, add Sichuan wax powder and Kollicoat MAE 100P into a three-dimensional mixer and mix for 15 minutes, and spread the mixture evenly on a stainless steel plate superior.
[0049] 2) Put the stainless steel plate from operation 1) into an oven and heat it to 100°C, continue heating for 2 hours, take out the stainless steel plate from the oven and cool it down to room ...
Embodiment 3
[0058] Preparation of Quetiapine Fumarate 50 mg, 200mg, 300mg, 400mg Sustained Release Tablets
[0059] prescription
quantity
content%
Quetiapine Fumarate
230.4 mg mg
38.40%
Kollicoat MAE 100P
69.6mg
11.60%
Sichuan wax powder
35mg
5.83%
hydrogenated castor oil
60mg
10.00%
180mg
30.00%
Micropowder silica gel
20mg
3.33%
5mg
0.84%
Total
600mg
100%
[0060] 1) Pass quetiapine fumarate through a 100-mesh sieve, pass hydrogenated castor oil through a 100-mesh sieve, add Sichuan wax powder and Kollicoat MAE 100P into a three-dimensional mixer and mix for 15 minutes, and spread the mixture evenly on a stainless steel plate superior.
[0061] 2) Put the stainless steel plate from operation 1) into an oven and heat it to 100°C, continue heating for 2 hours, take out the stainless steel plate from the oven and cool it down to r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com