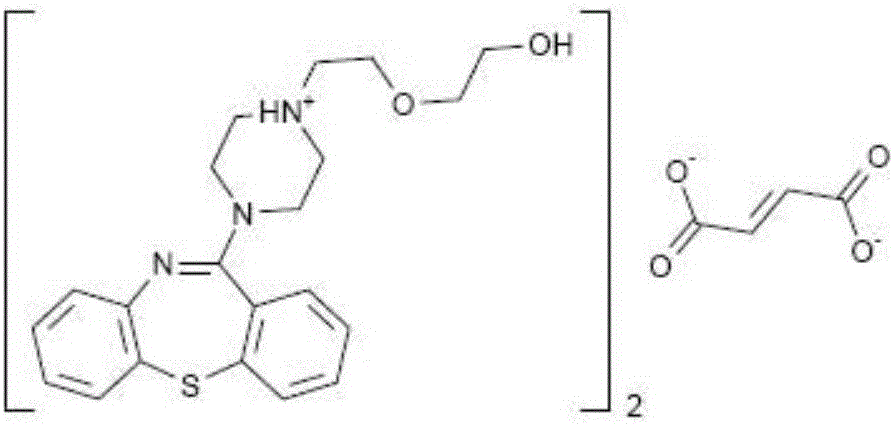
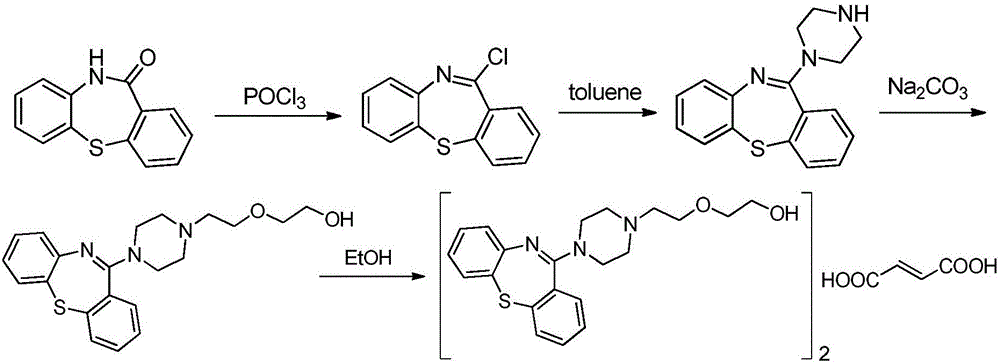
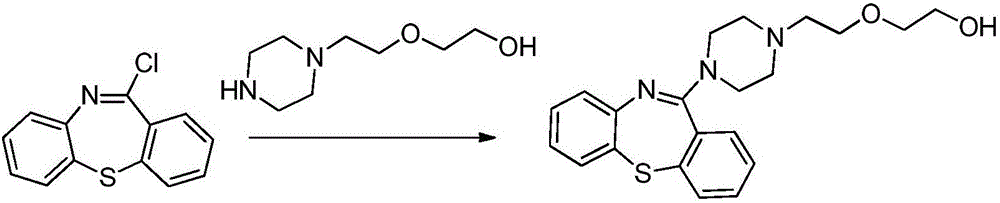
Quetiapine hemifumarate synthesis technology
A technology of quetiapine fumarate and synthesis process, applied in the direction of organic chemistry and the like, can solve problems such as unfavorable finished product quality control, a large amount of phosphorus-containing waste water, long production cycle, etc., and achieve easy industrial production, high yield, and product quality. excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048](1) Add 113g of isopropanol and 25ml of water into a No. 1 three-necked flask, add 50g of mercaptosalicylic acid and 51.1g of o-chloronitrobenzene under stirring, replace with nitrogen, the system is a cloudy liquid, and then add 32.43g of hydroxide Sodium and 33.1g water solution and 61g water; reflux reaction for 10-12h, TLC detection of mercaptosalicylic acid after the reaction is completed, cool down to 20-30°C, adjust pH<1 with concentrated hydrochloric acid, stir and crystallize for 1h, filter with suction, Washed twice with water and toluene respectively, and dried under reduced pressure at 55°C to obtain 80.3g of 2-(2-nitrophenylmercapto)benzoic acid (compound of formula (I)), with a molar yield of 90% and a purity of 98.5%.
[0049] (2) Add 10.0g of 2-(2-nitrophenylmercapto)benzoic acid and 80g of dichloromethane into No. 2 three-necked flask, add 8.3g of thionyl chloride dropwise, keep warm at 40°C for 4-5h, spin dry, The oil was diluted with 50g of dichloromet...
Embodiment 2
[0055] (1) Add 226g of isopropanol and 50ml of water into a 500ml No. 1 three-neck flask, add 100g of mercaptosalicylic acid and 102.2g of o-chloronitrobenzene under stirring, replace with nitrogen, the system is a turbid liquid, and then add 64.86g of The solution prepared by sodium hydroxide and 66.2g water and 122g water; reflux reaction for 10-12h, TLC detection of mercaptosalicylic acid after the reaction is completed, cool down to 20-30°C, adjust PH<1 with concentrated hydrochloric acid, stir and crystallize for 1h, pump Filter, wash with water and toluene twice respectively, and dry under reduced pressure at 55° C. to obtain 162.4 g of 2-(2-nitrophenylmercapto) benzoic acid (ie, the compound of formula (I)), with a molar yield of 91.0% and a purity of 98.8%.
[0056] (2) Add 40.0g of 2-(2-nitrophenylmercapto)benzoic acid and 320g of dichloromethane into No. 2 three-necked flask, add 33.2g of thionyl chloride dropwise, keep warm at 40°C for 4-5h, spin dry, The oil was di...
Embodiment 3
[0060] (1) Add 452g of isopropanol and 100ml of water into a 1000ml No. 1 three-neck flask, add 200g of mercaptosalicylic acid and 204.4g of o-chloronitrobenzene under stirring, replace with nitrogen, the system is a cloudy liquid, and then add 129.72g of The solution prepared by sodium hydroxide and 132.4g water and 244g water; reflux reaction for 10-12h, TLC detection of mercaptosalicylic acid after the reaction is completed, cool down to 20-30°C, adjust PH<1 with concentrated hydrochloric acid, stir and crystallize for 1h, pump Filter, wash with water and toluene twice respectively, and dry under reduced pressure at 55° C. to obtain 332.8 g of 2-(2-nitrophenylmercapto) benzoic acid (ie the compound of formula (I)), with a molar yield of 90.5% and a purity of 99.0%.
[0061] (2) Add 160.0g 2-(2-nitrophenylmercapto)benzoic acid (compound of formula (I)) and 1280g dichloromethane into No. 2 three-neck flask, add 132.8g thionyl chloride dropwise, and keep warm at 40°C React for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com