Preparation method of rupatadine fumarate tablet
A technology of rupatadine fumarate and magnesium stearate, which is applied in the field of medicine, can solve problems such as repeated and complicated operations, achieve stable and controllable processes, improve bioavailability, and increase solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Embodiment 1, preparation rupatadine fumarate tablet
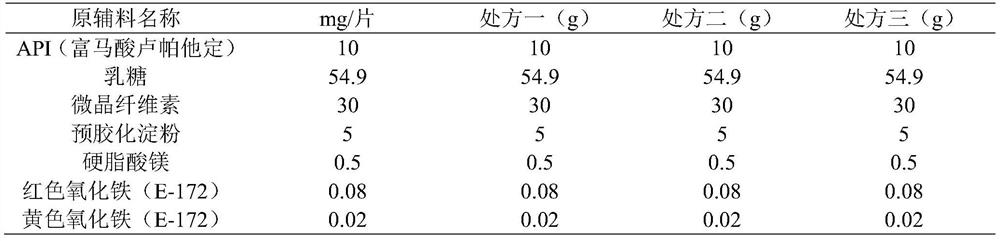
[0089] This embodiment provides the preparation of preparing 10,000 prescriptions, and the composition of the prescription is shown in Table 16:
[0090] Table 16 Prescription Composition of Rupatadine Fumarate Tablets
[0091]
[0092] Made into 10,000 pieces
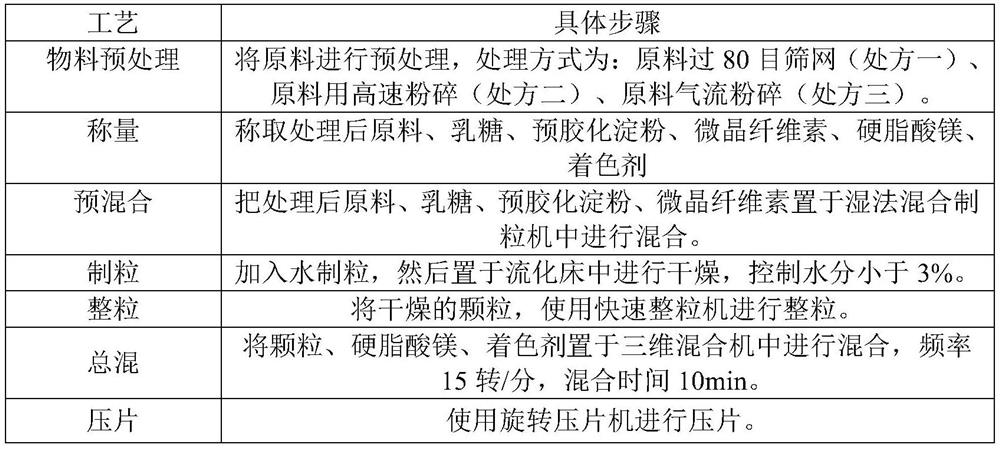
[0093] Prepared by wet granulation process, the process steps are as shown in Table 17:
[0094] Table 17 Wet granulation process of rupatadine fumarate tablets
[0095]
[0096] Concrete process conditions are as shown in table 18 and table 19:
[0097] Table 18 Conditions for wet granulation and fluid bed drying
[0098]
[0099] The specific parameters of tableting are shown in Table 19.
[0100] Table 19 Tablet Compression Conditions
[0101]
[0102] Dissolution data are shown in Table 20.
[0103] Table 20 Dissolution Data
[0104]
[0105]
[0106] By scaling up the determined crushing ratio, it was shown that the process had...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com