Preparation method of rupatadine fumarate
A technology of rupatadine fumarate and desloratadine, which is applied in the field of preparation of rupatadine fumarate, can solve the problems of low yield, low selectivity, and difficult separation, and achieve high yield , good reaction selectivity and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
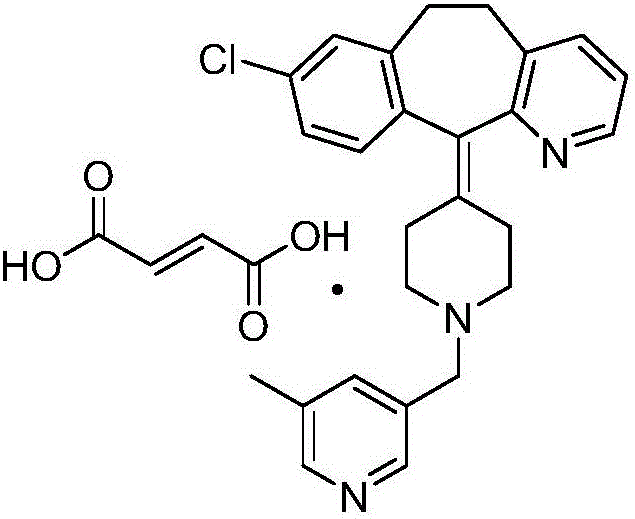
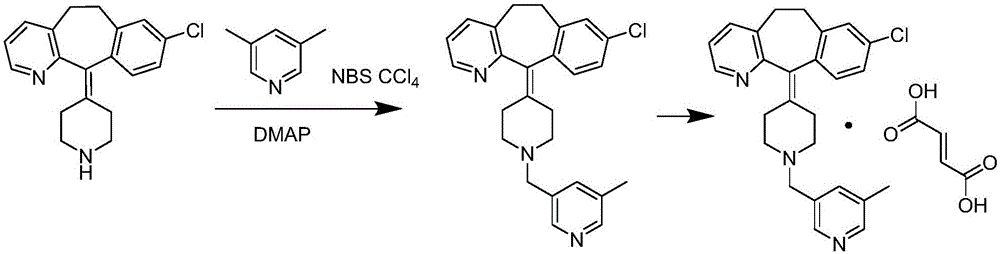
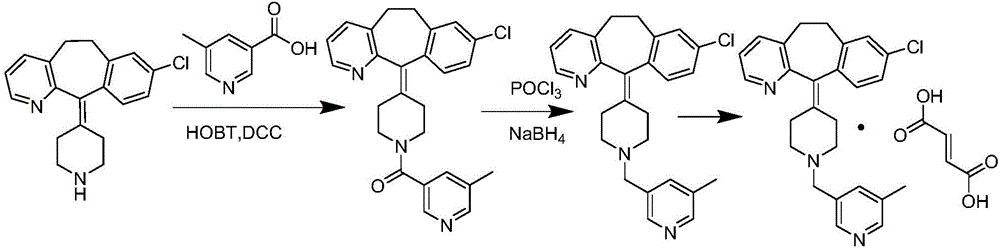
[0032] A preparation method of rupatadine fumarate shown in formula (VII), the preparation method comprising the steps of:
[0033] (1) using 5-methylnicotinic acid shown in formula (I) as a raw material, reacting in the presence of methanol and a catalyst to obtain the corresponding compound of formula (II);
[0034]
[0035] (2) the compound of formula (II) is reacted to obtain the corresponding compound of formula (III) in the presence of a reducing agent and a solvent;
[0036]
[0037] (3) the compound of formula (III) is reacted to obtain the corresponding compound of formula (IV) in the presence of a brominating agent and a solvent;
[0038]
[0039] (4) condensation of the compound of formula (IV) and desloratadine (V) under the action of an acid-binding agent and a solvent to obtain rupatadine (VI);
[0040]
[0041] (5) reacting rupatadine (VI) in the presence of fumaric acid and a solvent to obtain the corresponding rupatadine fumarate (VII);
[0042] ...
Embodiment 1
[0047]Add 20g of 5-methylnicotinic acid into 100mL of methanol, add 22mL of thionyl chloride dropwise at 20-30°C, heat up to reflux after the dropwise addition, stir for 2-3 hours, concentrate, add 50mL of water, dissolve and adjust with ammonia water pH to 8-9, extracted with ethyl acetate (250mL×2), combined organic phases, washed with saturated brine 100mL, dried the organic layer, concentrated to give 21.1g of compound (II), yield 95.5%; melting point: 44-45°C ; ESI-MS: m / z 151.95 ([M+H]+).
Embodiment 2
[0049] Add 10 g of compound (II) into 100 mL of methanol, dissolve and add 8.8 g of sodium borohydride, heat up to 55°C for 1 h, cool to room temperature, concentrate, add 10 mL of water, stir for 30 minutes, filter, and extract the filtrate with ethyl acetate ( 50 mL×2) twice, combined the organic layers, washed with 50 mL of saturated sodium chloride, dried the organic layer, and concentrated to obtain 7.5 g of compound (III). Yield 92.1%; MS-ESI (m / z): 124 (M)+.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com