Rupatadine fumarate compound
A technology of rupatadine fumarate, compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
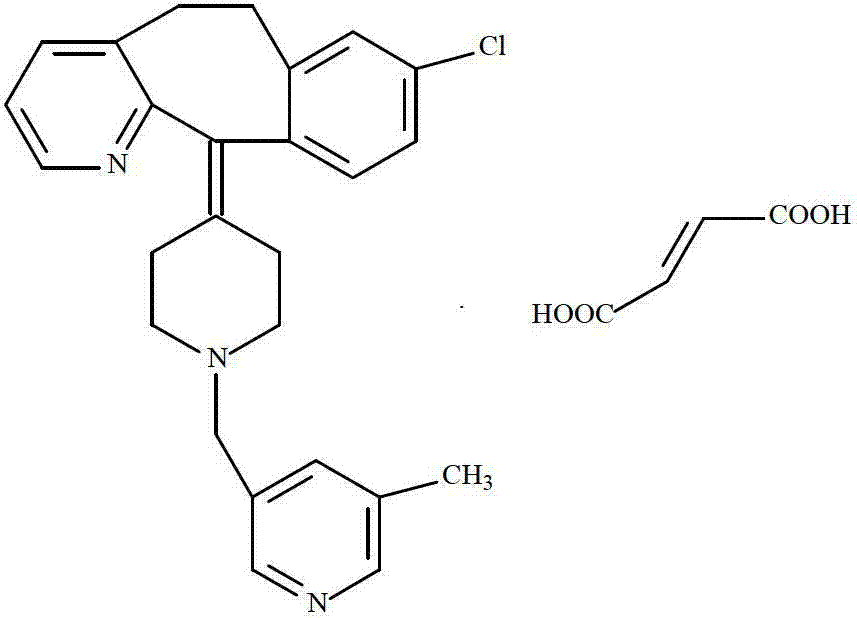
[0019] Using 3-chloromethyl-5-methyl-pyridine hydrochloride and deratadine as raw materials, using triethylamine as a base, reacting at room temperature in a mixed solvent of acetone and ethyl acetate (volume ratio 1:1) to obtain the free base, Salt-forming reaction of free base and fumaric acid in ethanol to obtain crude rupatadine fumarate, stirring and washing the crude product three times in a mixed solvent of acetone and ethyl acetate (volume ratio 1:5) to obtain the rupatadine fumarate of the present invention. compound.
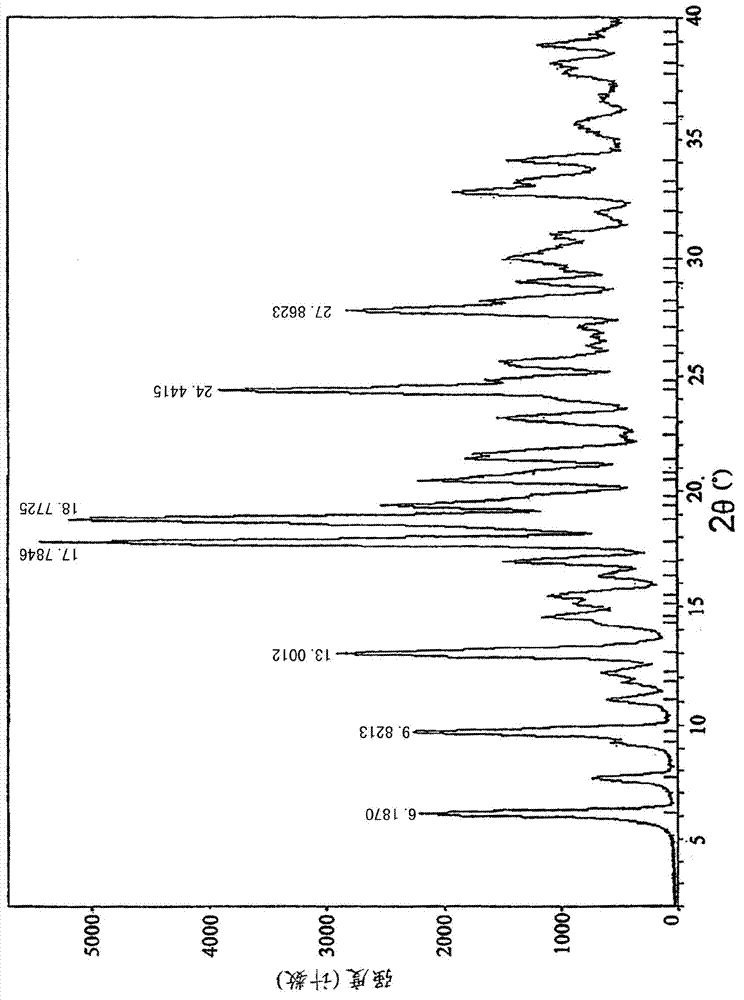
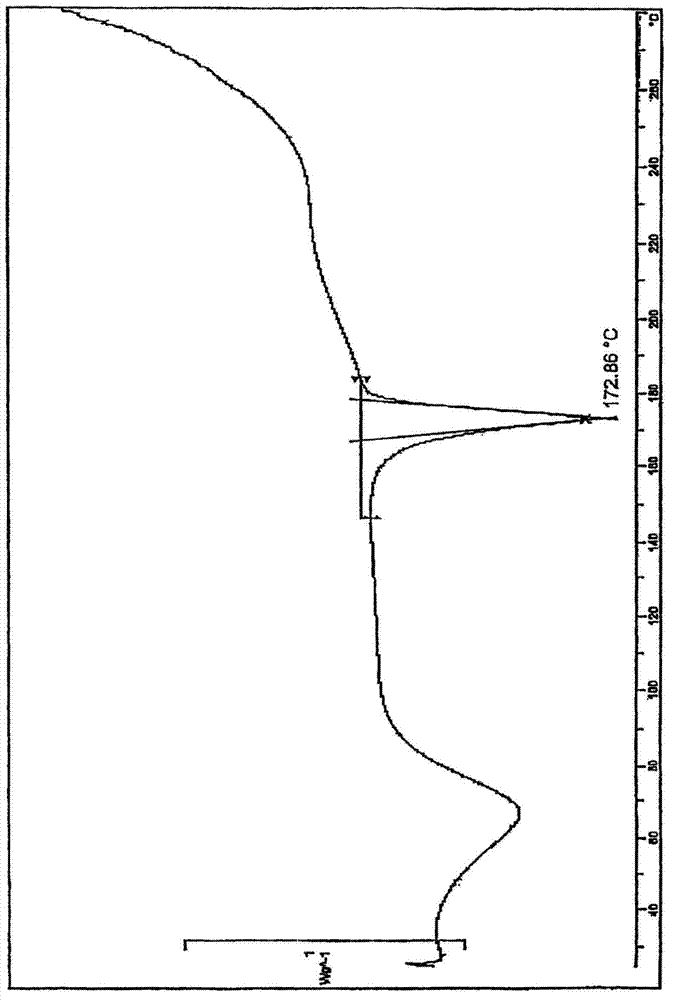
[0020] Powder X-ray diffraction: using Cu-K radiation, the X-ray powder diffraction (XRPD) spectrum of this compound is shown in the attached figure 1 . Differential scanning calorimetry: the differential thermal analysis (DSC) spectrum of this compound is shown in the attached figure 2 .
Embodiment 2
[0022] Using 3-chloromethyl-5-methyl-pyridine hydrochloride and deratadine as raw materials, using triethylamine as a base, reacting at room temperature in a mixed solvent of acetone and ethyl acetate (volume ratio 1.5: 1) to obtain the free base, Salt-forming reaction of free base and fumaric acid in ethanol to obtain crude rupatadine fumarate, stirring and washing the crude product twice in a mixed solvent of acetone and ethyl acetate (volume ratio 1:6) to obtain the present invention compound of. The crystal map is attached figure 1 and 2 .
Embodiment 3
[0024] Using 3-chloromethyl-5-methyl-pyridine hydrochloride and deratadine as raw materials, using triethylamine as a base, reacting at room temperature in a mixed solvent of acetone and ethyl acetate (volume ratio 1.5: 1) to obtain the free base, Salt-forming reaction of free base and fumaric acid in ethanol to obtain crude rupatadine fumarate, stirring and washing the crude product twice in a mixed solvent of acetone and ethyl acetate (volume ratio 1:8) to obtain the present invention compound of. The crystal form spectrum is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com