Liquid preparation for dosing eyes and method for making the same
A technology for liquid preparation and eye administration, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Preparation of Rupatadine Eye Drops
[0054] Main drug and excipient name dosage
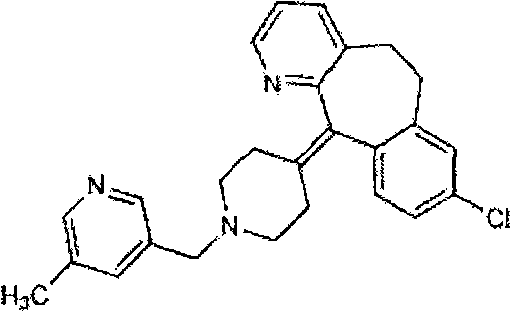
[0055] Rupatadine 0.625g
[0056] Borax 0.14g
[0057] Boric acid 0.10g
[0058] Ethylparaben 0.03g
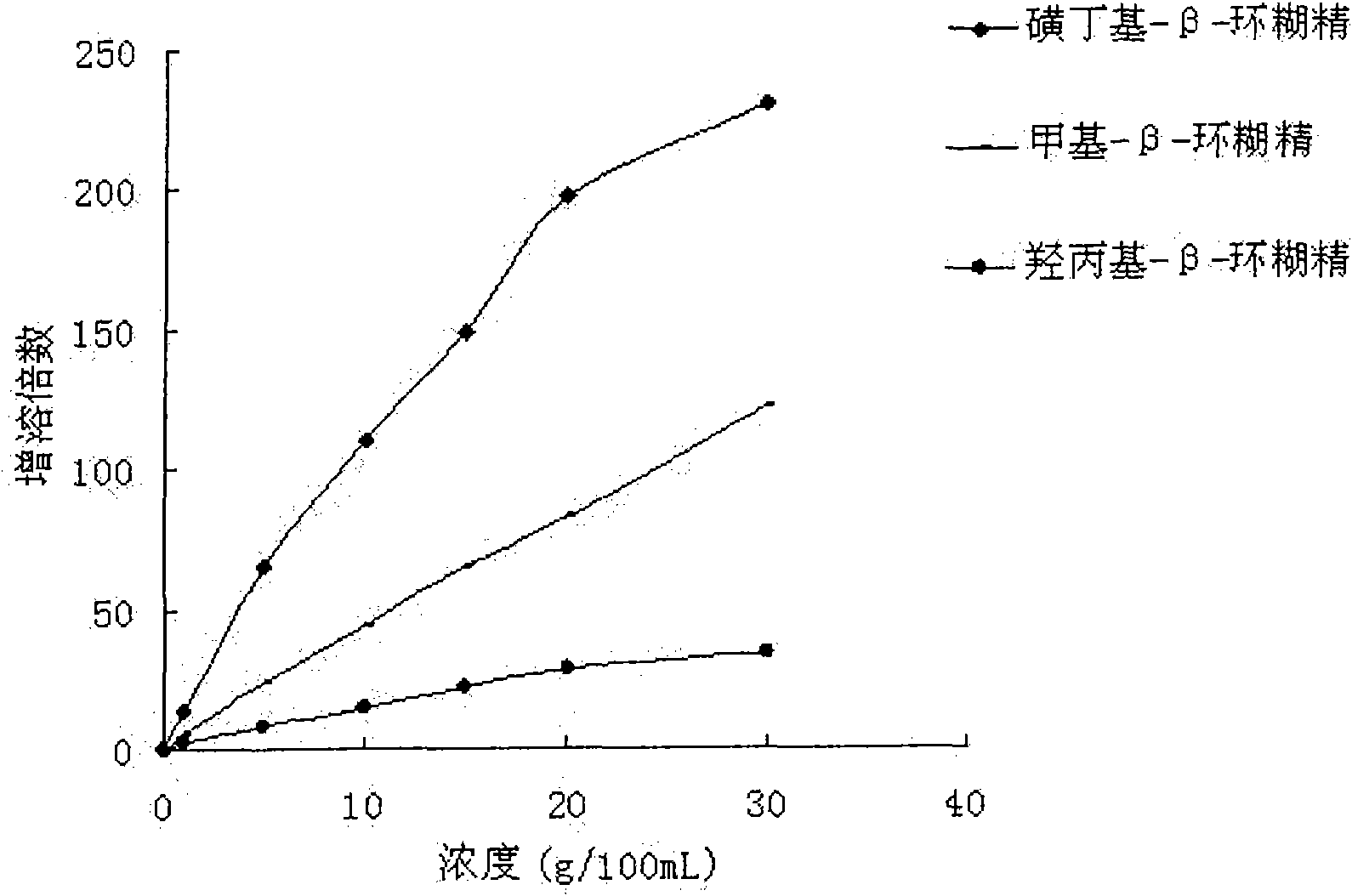
[0059] Sulfobutyl ether-β-cyclodextrin 1.50g
[0060] Distilled water to 100mL
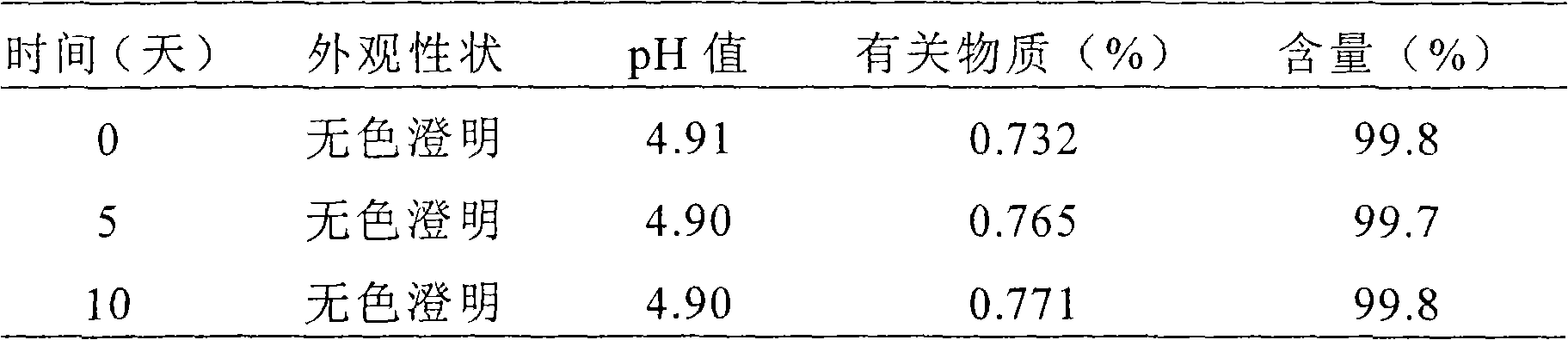
[0061] Preparation method: Dissolve borax, boric acid, sulfobutyl ether-β-cyclodextrin and ethylparaben in 90mL distilled water, add rupatadine to the above solution after crushing it through an 80-mesh sieve, and add distilled water to 100 mL, fully shake to dissolve rupatadine, the pH is between 4.5 and 5.0, and filter through a 0.45 μm microporous membrane to obtain rupatadine eye drops. Flow-through steam sterilizable, dispensed in multi-dose delivery devices.
Embodiment 2
[0062] Example 2 Preparation of Rupatadine Ophthalmic Gel
[0063] Main drug and excipient name dosage
[0064] Rupatadine 10.0g
[0065] Sulfobutyl ether-β-cyclodextrin 10.0g
[0066] Ethylparaben 0.03g
[0067] Boric acid 0.18g
[0068] Borax 0.12g
[0069] Carbopol 940 0.01g
[0070] Distilled water to 100mL
[0071] Preparation method: Sprinkle Carbopol 940 into 10mL of distilled water several times to make it slowly swell to obtain Carbopol 940 water dispersion. After crushing rupatadine through an 80-mesh sieve, add ethylparaben, borax, boric acid and sulfobutyl ether-β-cyclodextrin into 80mL water, shake fully to dissolve completely, and its pH is 5.0~ 5.5, and then added to Carbopol 940 aqueous dispersion, added distilled water to 100mL, and stirred evenly to obtain rupatadine ophthalmic gel. Flow-through steam sterilizable, dispensed in multi-dose delivery devices.
Embodiment 3
[0072] Example 3. Preparation of Rupatadine Eye Drops
[0073] Main drug and excipient name dosage
[0074] Rupatadine 0.20g
[0075] Hydroxypropyl-β-cyclodextrin 2.00g
[0076] Chlorobutanol 0.05g
[0077] Boric acid 0.10g
[0078] Borax 0.08g
[0079] Distilled water to 100mL
[0080] Preparation method: Dissolve chlorobutanol, borax, boric acid and hydroxypropyl-β-cyclodextrin in 90mL distilled water, add rupatadine to the above solution after pulverizing it through an 80-mesh sieve, and add distilled water to 100mL, shake fully to dissolve rupatadine, the pH is between 4.5 and 5.0, and filter through a 0.45μm microporous membrane to obtain rupatadine eye drops. Flow-through steam sterilizable, dispensed in multi-dose delivery devices.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com